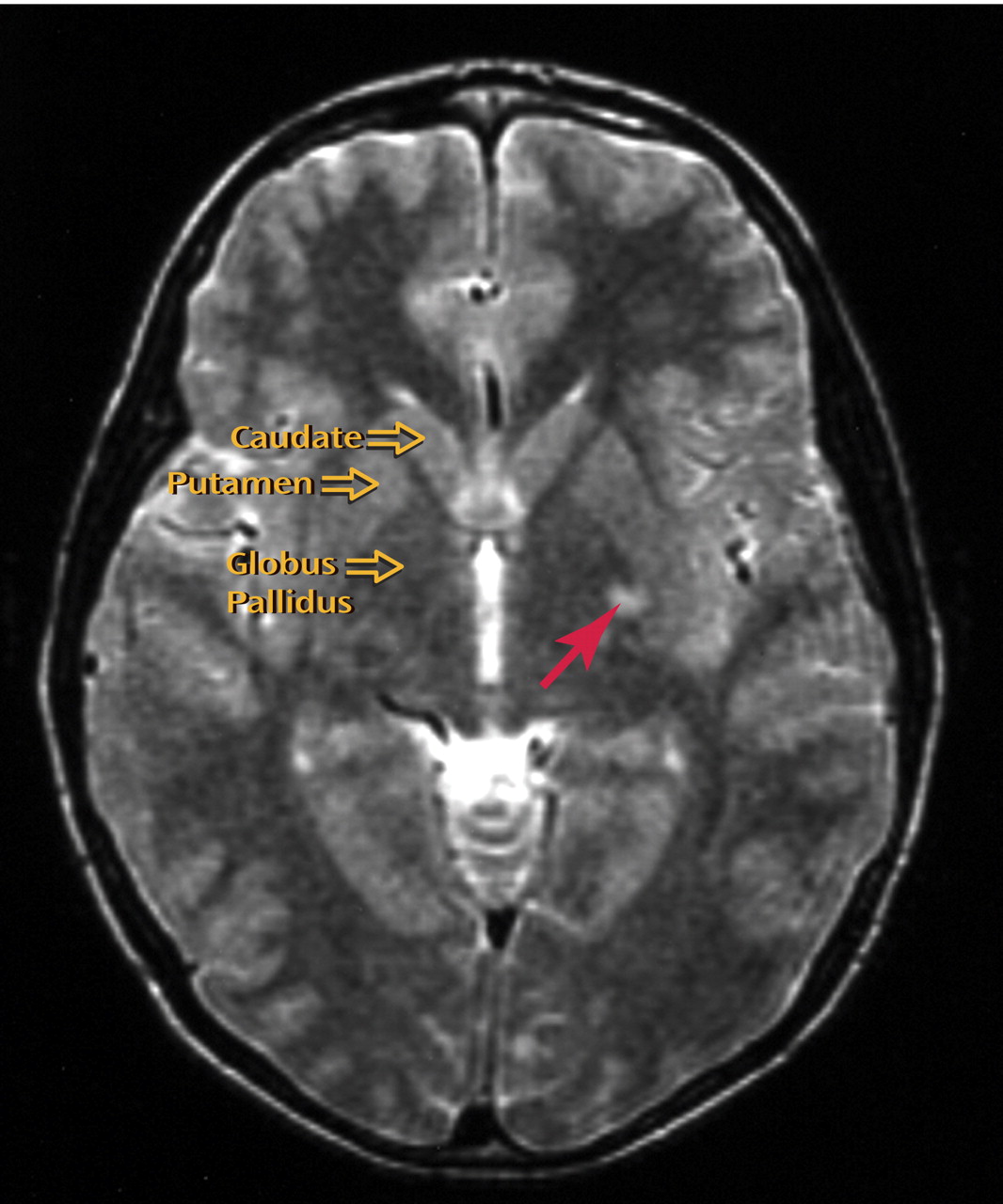
T 2 -weighted magnetic resonance imaging (MRI) allows detection of tissue abnormalities, including white matter and gray nuclei cerebral hyperintensities; these are bright patches in brain parenchyma that neither enhance with gadolinium contrast nor cause mass effects. Cerebral hyperintensities are believed to represent locally increased water content caused by increased microvascular permeability.
Cerebral hyperintensities often appear in the healthy adult population; however, histopathological and recent anatomopathological and angiographic evidence indicates that cerebral hyperintensities should not be considered a normal variant but rather a subclinical manifestation of small-vessel disease
(1,
2) . In populations at low risk for vascular disease, such as children and young adults, cerebral hyperintensities are most often associated with vasculitides or neuroinflammatory/neurodegenerative cerebral processes of diverse etiology
(2) . Cerebral hyperintensities are commonly found, for example, in the basal ganglia of children and young adults with Sydenham’s chorea
(3), a neuropsychiatric disorder thought to be a sequela of infection with group A beta-hemolytic streptococcus. Individuals with Sydenham’s chorea tend to have enlarged basal ganglia as well, presumably caused by edema from an autoimmune vasculitis
(4) .
Behavioral manifestations of Tourette’s syndrome, obsessive compulsive disorder (OCD), and attention deficit hyperactivity disorder (ADHD) closely resemble the hyperkinetic, choreiform movements of Sydenham’s chorea. Moreover, elevated titers of group A beta-hemolytic streptococcus antibodies have been detected in Tourette’s syndrome, OCD, and ADHD, although not in all studies
(5) . These elevated titers of group A beta-hemolytic streptococcus antibodies have been associated with basal ganglia enlargement in OCD and ADHD
(6) . In addition, children and adolescents with conduct disorder or ADHD were found to have significantly more cerebral hyperintensities relative to less psychiatrically ill comparison subjects
(7) .
Using healthy comparison subjects, we attempted to replicate the finding that ADHD is linked to more cerebral hyperintensities. Moreover, we aimed to expand this finding by assessing whether Tourette’s syndrome and OCD are also associated with an increased frequency of cerebral hyperintensities. In addition, we explored the association of cerebral hyperintensities with the size of the basal ganglia.
Method
The 132 participants, ages 7 to 18, included 100 children with Tourette’s syndrome, OCD, or ADHD and 32 comparison subjects. Patients were recruited through outpatient clinics or local support groups. Healthy subjects were recruited randomly from a telemarketing list of families in the local community. Parents of participants provided informed written consent; participants provided written assent. The institutional review board at Yale University School of Medicine approved the study. Subjects with Tourette’s syndrome, OCD, or ADHD met DSM-IV criteria for one or more of these diagnoses. Exclusionary criteria for patients included a major neuropsychiatric disorder that antedated onset of Tourette’s syndrome, OCD, or ADHD; head trauma; or IQ below 80. Comparison subjects had no current axis I disorder or history of tics, OCD, or ADHD. Severity of psychiatric symptoms was assessed in all participants by instruments described elsewhere
(6) .
MRI scans of the brain were obtained for all subjects using a 1.5T scanner (GE, Milwaukee). Two neuroradiologists, blind to diagnosis, assessed cerebral hyperintensities using a consensus procedure on axial and coronal proton-density and T
2 -weighted axial images (conventional spin echo, TR=3000 ms, TE=20/112 ms, 3 mm contiguous slices, 256×256 matrix, 24 cm field of view) (
Figure 1 ). Whole-brain volume and volumes of cortex and subcortex, including basal ganglia, thalamus, and brain stem, were defined using manual (basal ganglia) and semiautomated (cortex, subcortex) morphometric procedures described elsewhere on high-resolution T
1 -weighted 3D-SPGR images
(6) .
Logistic regression assessed the likelihood of detecting cerebral hyperintensities in the entire cerebrum, cortex, and subcortex of subjects with a neuropsychiatric disorder compared with healthy subjects. Whole-brain volume, age, and gender were entered as covariates in multivariate analysis. Similar models were used to compare the likelihood of detecting cerebral hyperintensities in the cortex and subcortex within groups, using cortical and subcortical volumes as covariates to control for the greater likelihood of detecting cerebral hyperintensities within the larger volume of the cortex. Analysis of covariance (ANCOVA) assessed the association of basal ganglia volumes with the number of cerebral hyperintensities in the patient group. Psychopharmacological treatment, comorbidity with other psychiatric diagnoses, and symptom severity were statistically nonsignificant when entered as covariates and were therefore excluded from the model. All p values were two-sided.
Results
Patients were significantly older than comparison subjects (11.4 [SD=2.6] years versus 10.0 [SD=1.7] years, t=2.87, df=130, p=0.006), but group gender ratios were similar (male:female=78:22 versus 19:13, χ 2 =3.41, p=0.07). In the 100 patients, 62 had Tourette’s syndrome, 28 had OCD, 45 had ADHD, and 29 had more than one diagnosis. Gender was unequally distributed in all three disorders and in subgroups with and without comorbidity, respectively: Tourette’s syndrome (male:female, 9/51, 5/28), OCD (male:female, 10/18, 7/5), and ADHD (male:female, 7/39, 6/20).
We detected 41 cerebral hyperintensities in 30 (30.0%) patients (nine in the cortex, three in the cerebellum, and 29 in the subcortex [nine in the basal ganglia]), relative to eight cerebral hyperintensities in five (15.6%) comparison subjects (five in the cortex, three in the subcortex [one in the basal ganglia]), a statistically significant difference (OR=4.2, 95% confidence interval [CI]=1.3–13.9, p=0.01). The cerebral hyperintensities prevalence rate in healthy subjects was consistent with that of previous, smaller studies (5%-30%). Likewise, age but not gender was a significant cofactor in our study.
The three diagnoses were then separately matched against comparison subjects. Cerebral hyperintensities were detected significantly more often in children with Tourette’s syndrome (17/62 [28.3%], odds ratio=4.6, CI=1.2–17.2, p=0.02) and ADHD (19/45 [42.2%], odds ratio=4.8, CI=1.3–20.0, p=0.01) and more often, albeit nonsignificantly, in children with OCD (6/28 [21.4%], odds ratio=4.2, CI=0.9–20.2, p=0.10). Considering each diagnosis without comorbidity, cerebral hyperintensities were more frequent in Tourette’s syndrome (9/33 [27.3%], odds ratio=3.7, CI=0.9–15.2, p=0.08), OCD (2/12 [16.7%], odds ratio=4.1, CI=0.4–42.2, p=0.26), and ADHD (9/26 [34.6%], odds ratio=4.6, CI=1.2–18.1, p=0.03), although differences were statistically significant only for ADHD. Lack of statistical significance for “pure” Tourette’s syndrome and OCD groups is likely attributable to smaller sample size and thus reduced statistical power. Nonetheless, the odds ratios for these two groups are similar in magnitude to the odds ratios for the other groups assessed.
Cerebral hyperintensities were detected significantly more often in the subcortex of patients than in comparison subjects (29/98 [29.6%] versus 2/30 [6.7%], odds ratio=6.9, CI=1.2–40, p=0.03). However, the frequency of cortical cerebral hyperintensities frequency did not differ significantly between the two groups (8/89 [10.1%] versus 2/25 [8.0%], odds ratio=4.7, CI=0.5–42.7, p=0.16). Furthermore, patients were significantly more likely to have subcortical than cortical cerebral hyperintensities (29/98 [29.6%] versus 8/89 [9.0%], odds ratio=2.9, CI=1.2–7.0, p=0.01). Regional specificity was absent in comparison subjects (2/30 [6.7%] versus 2/25 [8.0%], odds ratio=2, CI=0.1–24.3, p=0.56). Finally, the frequency of cerebral hyperintensities in the basal ganglia of patients did not correlate significantly with basal ganglia volume, nor did mean basal ganglia volume correlate significantly with the presence of cerebral hyperintensities.
Discussion
Cerebral hyperintensities were significantly more abundant in the subcortex of children and adolescents with Tourette’s syndrome, OCD, or ADHD compared with healthy subjects. All three disorders seemed to contribute almost equally to this overall effect. The number of cerebral hyperintensities in the basal ganglia did not correlate significantly with basal ganglia volume.
Evidence suggests that autoimmune processes following group A beta-hemolytic streptococcus infection may contribute to the pathogenesis of a small proportion of cases of Tourette’s syndrome, ADHD, and OCD
(5) . Moreover, elevated serum titers of antistreptolysin-O (marker of acute infection) but not anti-DNase B (marker of chronic infection) antibodies have been associated with enlarged basal ganglia in ADHD children
(6) . This finding suggests that autoimmune processes may injure the basal ganglia and then presumably produce swelling and edema following acute infection. Likewise, the absence of a significant correlation of basal ganglia volume with the presence of cerebral hyperintensities in the basal ganglia suggests that if an inflammatory process following group A beta-hemolytic streptococcus infection is responsible for the cerebral hyperintensities, then the inflammation is likely to be restricted to the vascular bed after the acute initial parenchyma edema subsides, as appears to be the case in Sydenham’s chorea
(4) . Our limited statistical power to detect this correlation, given the relatively low frequency of cerebral hyperintensities in the basal ganglia of this sample, also likely contributed to the negative finding.
Neuroimaging studies increasingly document the presence of structural and functional abnormalities in the cortex and subcortex of persons with Tourette’s syndrome, OCD, and ADHD, suggesting that disturbances of cortical-subcortical connectivity may contribute to behavioral dysregulations (impulsivity, compulsion, and tics). A single insult to the basal ganglia may affect more than one of these anatomically and functionally distinct yet partially overlapping cortico-striato-thalamo-cortical circuits, contributing to the high comorbidity among these disorders found in the general population. Findings in the present study therefore support the notion that subcortical injury (possibly of an inflammatory autoimmune nature) contributes to the pathogenesis of Tourette’s syndrome, OCD, and ADHD in some individuals.
To investigate this possibility further, magnetic resonance (MR) spectroscopy could be used to assess neuronal viability and the molecular bases of tissue damage in the subcortical nuclei of patients with Tourette’s syndrome, OCD, and ADHD, and MR angiography may also be beneficial in assessing the presence of vasculitides in these populations. Future studies should also measure antineuronal, antinuclear, and antistreptococcal antibodies in conjunction with cerebral hyperintensities to better clarify whether subcortical cerebral hyperintensities in individuals with Tourette’s syndrome, OCD, and ADHD are associated with an autoimmune process.