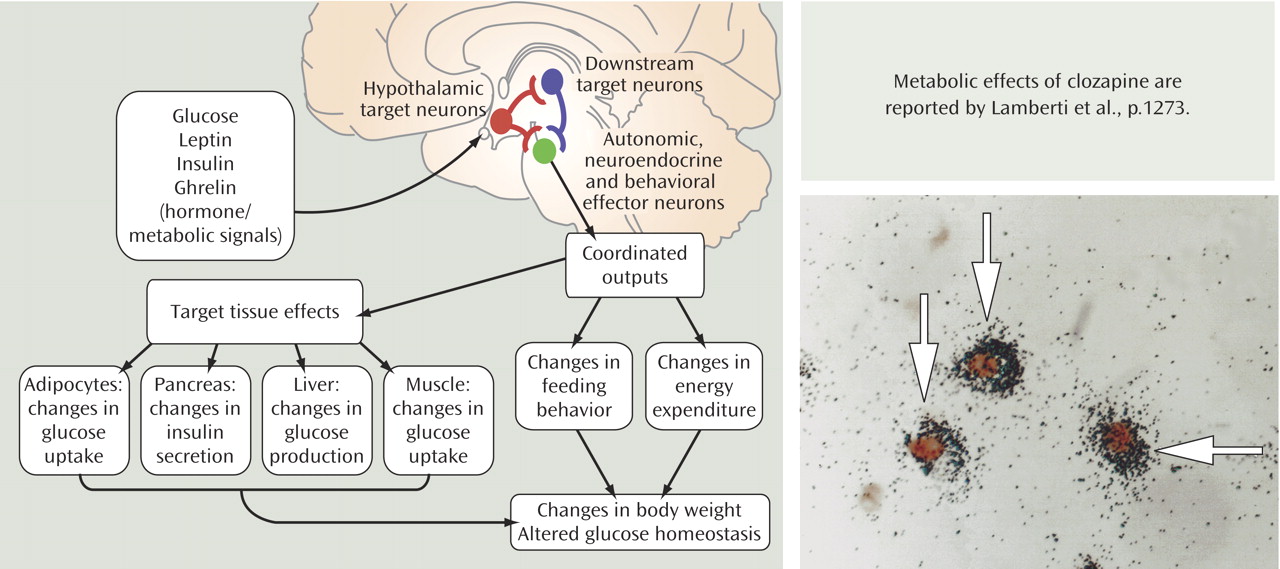
The control of body weight and energy homeostasis in humans has received much attention in recent years because of the rising rates of obesity. The field of psychiatry has become interested in these processes, since many of our medications perturb neurotransmitter systems regulating feeding mechanisms and result in weight gain and often diabetes. In the past decade, the understanding of the brain sites controlling body weight and glucose homeostasis has increased dramatically. One key site is the arcuate nucleus of the hypothalamus because it integrates peripheral signals of energy balance (shown in diagram). For example, leptin (from the Greek
leptos, meaning thin) is a circulating hormone produced by white adipose tissue that regulates feeding behavior. Exogenous leptin reduces appetite and feeding; leptin deficiency (both mice and humans with mutations in the leptin gene) causes extreme obesity. Leptin directly activates proopiomelanocortin (POMC) cells in the arcuate nucleus (shown on the right) to increase the release of melanocortin peptides, including the POMC product α-melanocyte stimulating hormone (α-MSH). Melanocortins (MC) inhibit food intake and regulate metabolism, energy storage, insulin secretion, and gastrointestinal motility predominantly via projections to MC
4 receptor neurons. Moreover, leptin also directly inhibits arcuate neurons, which produce agouti-related protein and neuropeptide Y. Agouti-related protein is an endogenous antagonist of α-MSH at MC
4 receptors. Another key metabolic signal that acts directly on the melanocortin circuit in the arcuate nucleus is the hormone ghrelin. During periods of reduced calorie availability, the stomach also increases the release of ghrelin before meals. Elevated ghrelin in the presence of low leptin levels induces appetite and results in obesity (e.g., ghrelin levels are high in Prader-Willi syndrome). Activation of ghrelin receptors in the arcuate nucleus is thought to activate neuropeptide Y and agouti-related protein neurons to promote weight gain. Of particular interest to psychiatrists is that serotonergic neurons innervate the hypothalamus, including POMC, neuropeptide Y, and agouti-related protein neurons. One key serotonin (5-HT) receptor that regulates body weight is the 5-HT
2C receptor. These receptors, expressed by POMC neurons in the arcuate, are required for the anorectic effect of
d -fenfluramine. Thus, antagonism of 5-HT
2C receptors by second-generation antipsychotics represents a potential mechanism for the weight gain and dysregulation of glucose homeostasis associated with these agents.