The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia project was initiated by the National Institute of Mental Health (NIMH) to determine the comparative effectiveness of antipsychotic drugs in typical clinical settings and situations. Unlike most clinical drug trials, in which patients who discontinue their initial treatment are dropped from the study, the CATIE study design allowed for patients who discontinued one study antipsychotic drug to enter subsequent phases of the study and receive another antipsychotic drug. Thus, the study mirrored real-world practice, in which clinicians must select among multiple alternatives for schizophrenia patients who do not respond to or do not wish to continue an antipsychotic treatment.
Results of several phases of the CATIE study, including the initial random assignment (to olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone—phases 1 and 1A) and the efficacy and tolerability arms of the second phase (phases 2E and 2T) have been previously reported
(1 –
3) . In this article we present results of another randomized phase of the study (phase 1B), which compared treatment with olanzapine, quetiapine, and risperidone among patients who had just discontinued the older antipsychotic drug perphenazine. The hypothesis during development of the CATIE protocol was that patients assigned to perphenazine in phase 1 would have a higher discontinuation rate than patients assigned to any of the newer drugs. Phase 1B was included to ensure that all individuals who entered phase 2 of the study had received a trial of a newer antipsychotic within the context of the CATIE schizophrenia study. Results of phase 1B are reported here.
Method
Study Setting and Design
The goal of the CATIE schizophrenia study was to determine the comparative effectiveness of antipsychotic drugs. Its rationale, design, and methods were previously described in detail
(1,
4 –
7) . The study was conducted between January 2001 and December 2004 at 57 U.S. clinical sites.
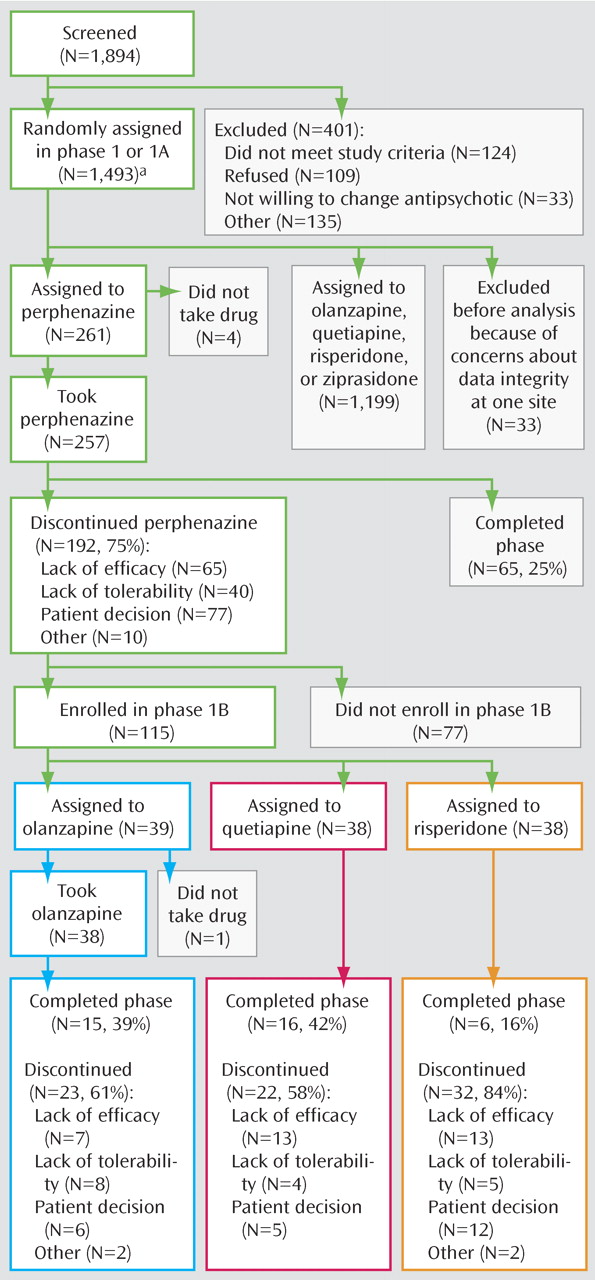
Figure 1 details the treatment assignments in phase 1, phase 1A, and phase 1B. Patients were initially randomly assigned to receive olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone under double-blind conditions and followed for up to 18 months or until treatment was discontinued for any reason (phases 1 and 1A). Phase 1A consisted of patients with tardive dyskinesia, who were excluded from assignment to perphenazine. Patients who were assigned to treatment in phase 1 with perphenazine and who discontinued it then entered phase 1B and received randomized, double-blinded treatment with olanzapine, quetiapine, or risperidone
(5) . If patients discontinued the assigned treatment in phase 1B, they were then eligible for phase 2.
In phase 2, patients and their study doctors could choose between two randomization pathways. The “efficacy” pathway (phase 2E), recommended to individuals who discontinued the previous treatment because of inefficacy, compared open-label clozapine to double-blinded treatment with olanzapine, quetiapine, and risperidone. The “tolerability” pathway (phase 2T), recommended to individuals who discontinued the previous treatment because of intolerability, compared double-blinded treatment with olanzapine, quetiapine, risperidone, and ziprasidone.
This report is of results from phase 1B. Patients were randomly assigned to olanzapine, quetiapine, and risperidone in a 1:1:1 ratio. If the assigned treatment worked adequately, patients could continue taking this second study antipsychotic until the completion of 18 months of study treatment.
Participants
The initial inclusion criteria required an age of 18 to 65 years, a diagnosis of schizophrenia (determined by the Structured Clinical Interview for DSM-IV), and appropriateness for oral antipsychotic medication. The exclusion criteria were a diagnosis of schizoaffective disorder; diagnosis of mental retardation or other cognitive disorder; past serious adverse reaction to any of the proposed treatments; first episode of schizophrenia; history of treatment resistance, defined by persistence of severe symptoms despite an adequate trial of one of the proposed treatments or prior treatment with clozapine for treatment resistance; current pregnancy or breast-feeding; or serious and unstable medical condition. Patients with tardive dyskinesia at the time of study entry were not assigned to perphenazine in phase 1; therefore, no patients who entered the study described here had tardive dyskinesia when they first entered the study.
The study was approved by an institutional review board at each site, and written informed consent was obtained from each patient or the patient’s legal guardian.
Interventions
Identical capsules contained 7.5 mg of olanzapine, 200 mg of quetiapine, or 1.5 mg of risperidone. The medications were flexibly dosed within a range of one to four capsules daily, on the basis of the study doctor’s judgment. Overlap in the administration of the antipsychotic that the patient received in the prior phase was permitted for the first 4 weeks to allow for gradual transition to the new medication. Concomitant medications were permitted throughout the trial, except additional antipsychotics. The patients had monthly visits with study doctors.
The drug package insert for quetiapine specifies that it is to be given twice daily, while olanzapine and risperidone may be given once daily. To protect blinding, one-half of the patients taking olanzapine and risperidone were assigned to twice-daily dosing and the other half were assigned to once-daily dosing. To minimize initial side effects, patients assigned to quetiapine began treatment by receiving one 100-mg capsule on days 1 and 2, one twice daily on day 3, and one for the first dose of day 4. All patients assigned to twice-daily dosing received five identical-appearing capsules to begin treatment.
Objectives and Outcomes
Our objective was to examine possible differences in the overall effectiveness of olanzapine, quetiapine, and risperidone among this group of individuals with chronic schizophrenia who had just discontinued perphenazine. The primary outcome measure was time until treatment discontinuation due to all causes; this discrete outcome was selected because stopping or changing medication is a frequent occurrence and major problem in the treatment of schizophrenia. Medication discontinuation integrates patient and clinician judgments of efficacy, safety, and tolerability into a global measure of effectiveness and signals the need for a new treatment strategy. The key secondary outcome was the reason for treatment discontinuation as judged by the study doctor. Additional secondary efficacy outcomes included scores on the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression scale (CGI), which were collected at study baseline and after 1, 3, 6, 9, 12, 15, and 18 months of study participation, as well as any visit in which there was a transition from one phase to another. Secondary safety and tolerability outcomes included incidence of serious adverse events, incidence of treatment-emergent adverse events, and changes in weight, measures of neurologic side effects, and laboratory analytes.
Statistical Methods
Randomly assigned patients who received at least one dose of study medication constituted the intent-to-treat cohort. Time from the beginning of phase 1B until treatment discontinuation was estimated by Kaplan-Meier survival curves. The olanzapine, quetiapine, and risperidone treatment groups were compared by means of Cox proportional hazards regression models
(8) adjusting for whether the patient had had an exacerbation in the 3 months before entering the study as well as for race (white versus nonwhite), because of a slight imbalance at the phase 1B baseline. The overall difference among the three treatments was evaluated by using a test with 2 degrees of freedom. If significance was reached at a level of p<0.05, then pairwise comparisons of the treatment groups were also evaluated for statistical significance relative to p<0.05. Similar analyses were conducted for time until phase 1B discontinuation due to lack of efficacy, intolerability, and patient decision. For these analyses, patients discontinuing for any other reason were censored at the time of discontinuation.
The treatment groups were compared for change from phase 1B baseline in PANSS and CGI severity scores at months 3, 6, and 9 by using an analysis of covariance (ANCOVA) adjusting for whether the patient had had an exacerbation in the 3 months before entering the study and for the baseline value. Time was classified into quarterly intervals of phase 1B treatment, represented by months 3, 6, 9, 12, and 15. Any end-of-phase assessment falling within the same quarterly interval as a scheduled assessment was assigned to the next interval. Month 15 was excluded from statistical testing because of the small number of subjects.
The treatment groups were compared for baseline characteristics with an analysis of variance (ANOVA) or chi-square test. Overall treatment comparisons for the safety outcomes are presented for descriptive purposes. The p values are based on Poisson regression or ANCOVA, both of which adjusted for differential duration of treatment with the phase 1B study drugs. Fisher’s exact test was used in cases of a small number of subjects. For laboratory measures, exposure-adjusted ANCOVA least-squares means are presented, but because of skewed distributions, the p values are from a rank ANCOVA.
NIMH funded the study. The pharmaceutical companies whose drugs were included donated the drugs and provided input on the dose range only for their own drug; they had no other input in study design, analyses, or interpretation of results. The manuscript was written solely by the listed authors.
Results
Patient Characteristics and Disposition
Of the 1,493 patients who were enrolled in the study and randomly assigned to treatment in phase 1 or 1A, 257 were assigned to perphenazine and took at least one dose. Of the 192 patients who discontinued perphenazine in phase 1 and thus were eligible for phase 1B, 115 (60%) accepted random assignment in phase 1B, and 114 of these took at least one dose of the assigned medication.
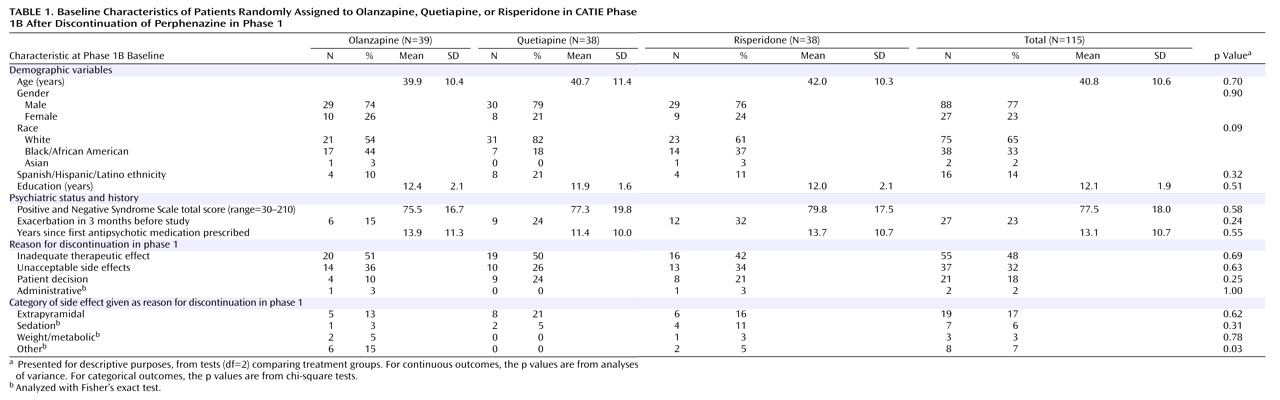
The demographic and clinical characteristics of the study group at the beginning of phase 1B are shown in
Table 1 . The characteristics of patients who entered phase 1B were generally representative of the original phase 1 study group, and the treatment groups did not differ significantly. However, most patients who discontinued perphenazine in phase 1 because of inefficacy (55 of 65, 85%) or intolerability (37 of 40, 93%) continued to phase 1B, while only a low proportion of patients who discontinued phase 1 because of “patient decision” continued (21 of 77, 27%).
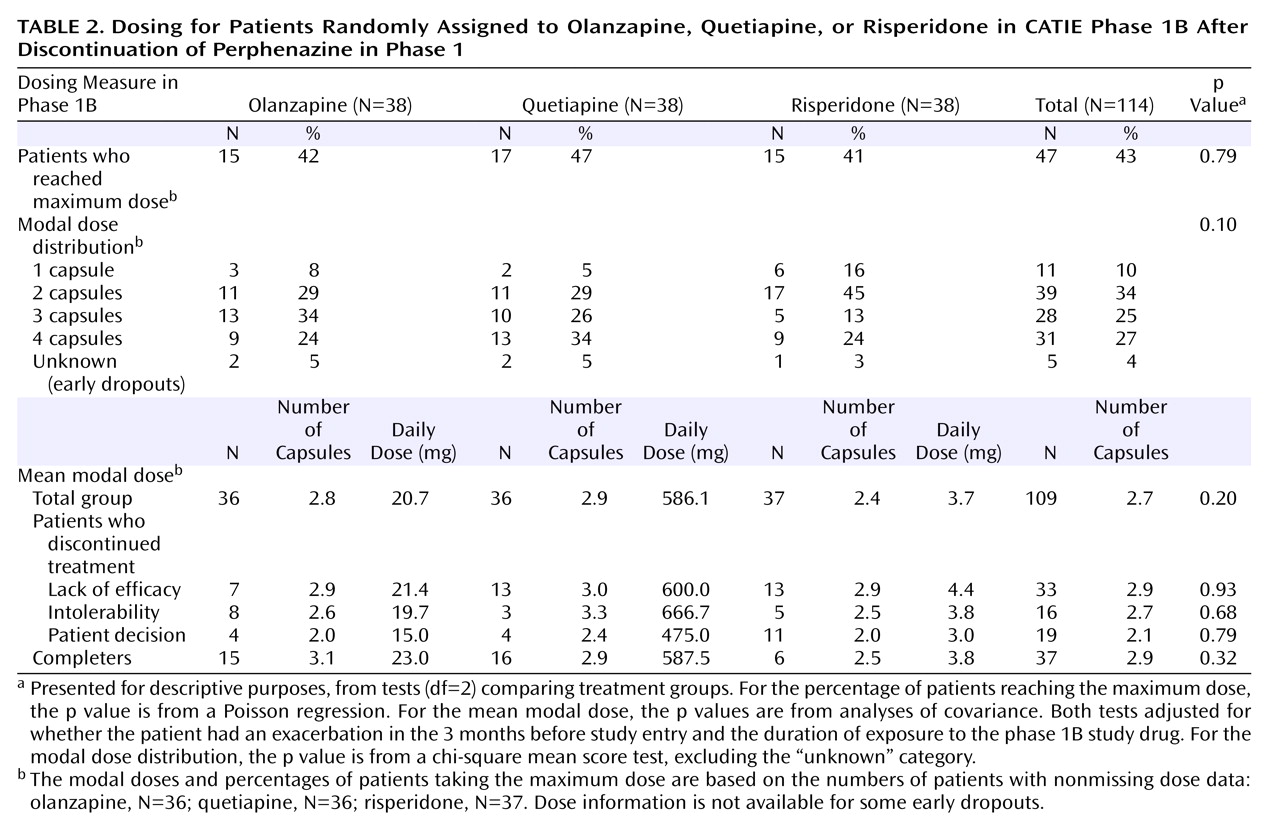
The mean modal doses and numbers of capsules are shown in
Table 2 ; the numbers of capsules for the three drugs were not significantly different. The dose ranges were as follows: olanzapine, 7.5–30.0 mg/day; quetiapine, 200–800 mg/day; and risperidone, 1.5–6.0 mg/day.
Treatment Discontinuation
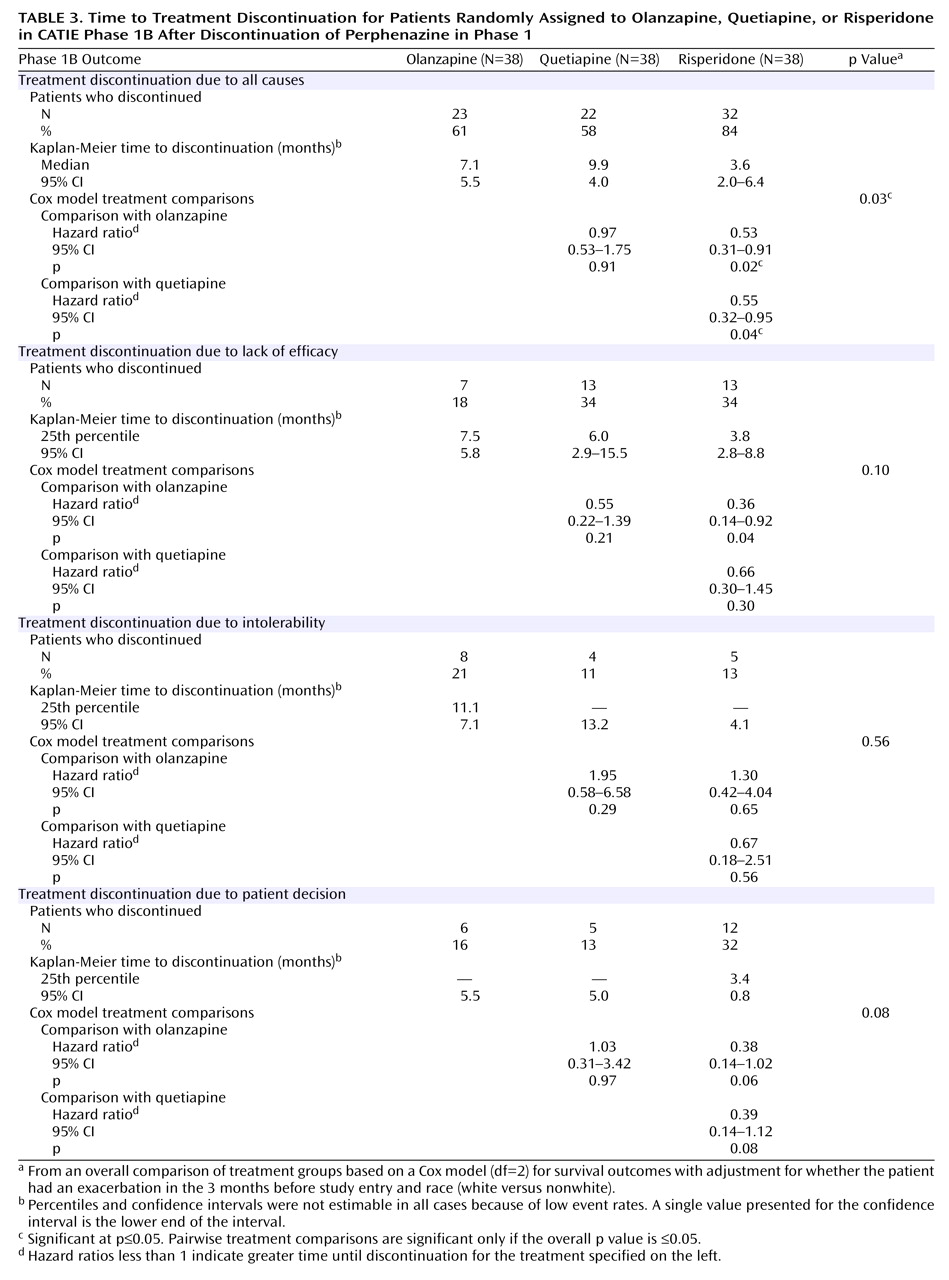
In phase 1B, 77 of the 114 patients (68%) in the intent-to-treat cohort discontinued treatment before completion of the study. The median treatment duration was 5.8 months.
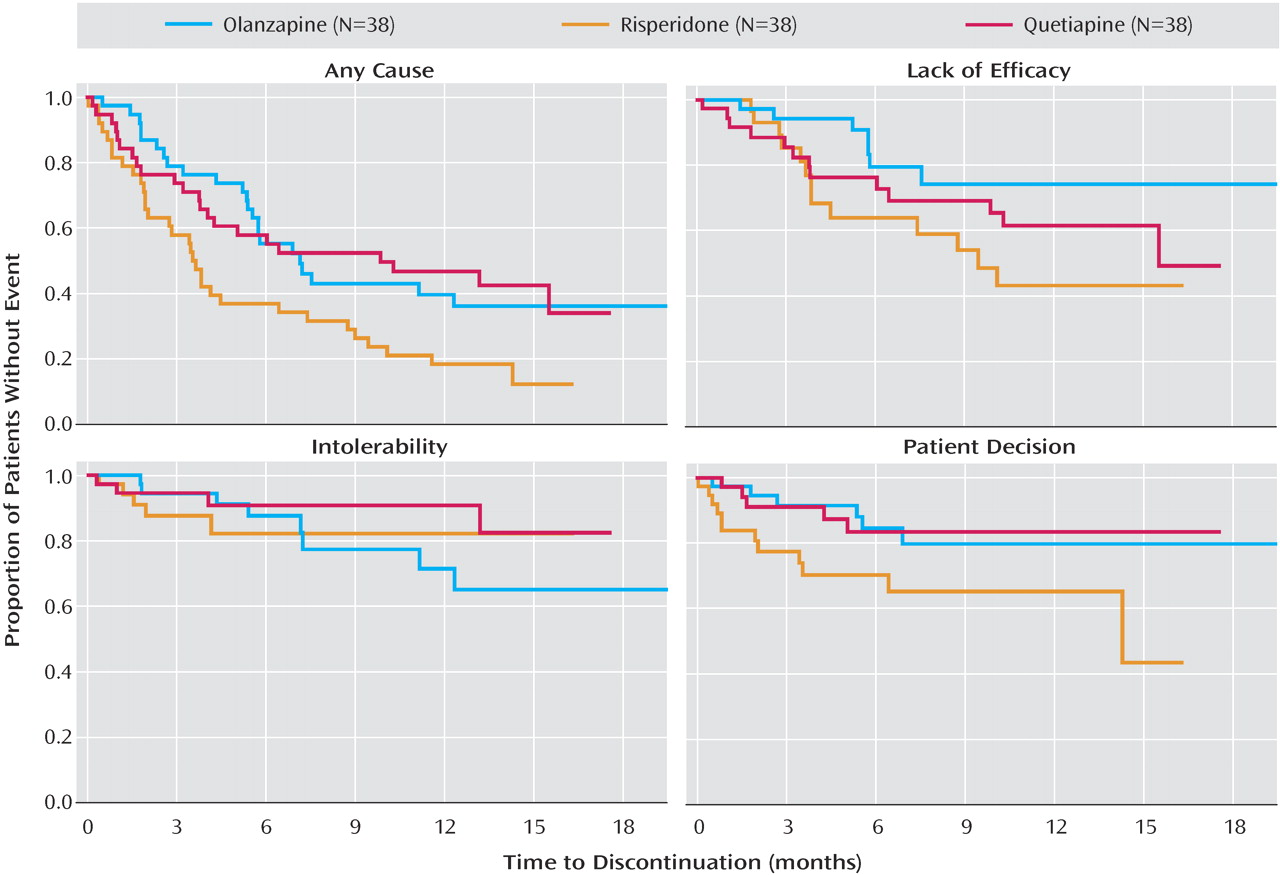
Discontinuation outcomes are presented in
Figure 2 and
Table 3 . The time to discontinuation for all causes was significantly longer for quetiapine and olanzapine than for risperidone. There was no significant difference between olanzapine and quetiapine.
There were no overall treatment group differences for time to discontinuation due to lack of efficacy, intolerable side effects, or patient decision (
Table 3 ).
In order to better understand the primary results, we examined subgroups. Among the 55 patients who had stopped taking perphenazine because of inefficacy, the lowest rate of discontinuation for all causes was among those assigned to olanzapine (five of 20, 25%), compared to nine of 19 (47%) who stopped taking quetiapine and eight of 16 (50%) who stopped taking risperidone.
Among the 37 patients who had discontinued perphenazine because of intolerability, the proportions of subjects who discontinued the assigned treatments in phase 1B for any reason were as follows: four of 10 (40%) for quetiapine, 10 of 14 (71%) for olanzapine, and 11 of 13 (85%) for risperidone. In addition, none of the 10 people in this group who were assigned to quetiapine discontinued it because of intolerability, compared to nine of 14 (64%) taking olanzapine and nine of 13 (69%) taking risperidone. Among the 19 people who discontinued perphenazine because of extrapyramidal symptoms, five of six (83%) discontinued risperidone for any reason, compared to two of five (40%) taking olanzapine and three of eight (38%) taking quetiapine.
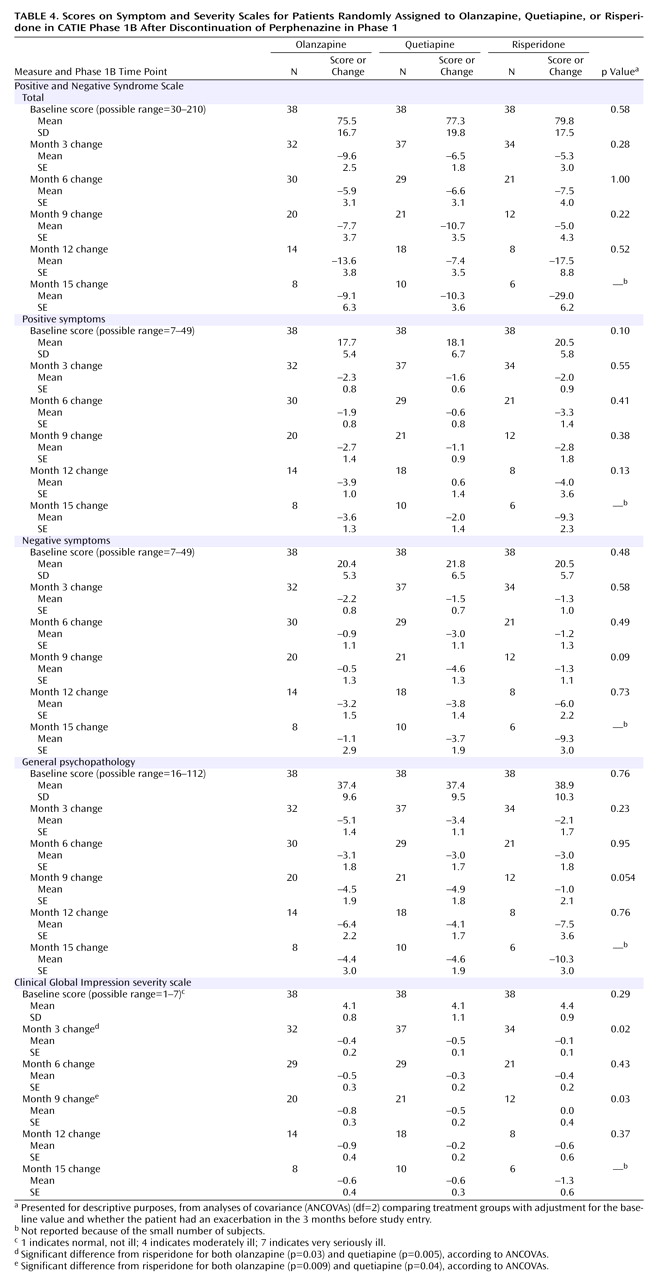
Efficacy Measures
The results of the PANSS and CGI analyses are presented in
Table 4 . There were no notable differences in the PANSS total or subscale scores among the treatment groups at any time point.
At 3 months and 9 months, for patients who remained in phase 1B there were slightly greater reductions in the CGI global severity measurement for patients treated with olanzapine and quetiapine than for those treated with risperidone, but differences were not present at 6 or 12 months.
Dose and Discontinuation Rates
We examined the modal doses according to the subjects’ reasons for leaving the study phase and found that the lowest mean modal doses for each drug were reached by those who discontinued treatment because of patient decision (
Table 2 ).
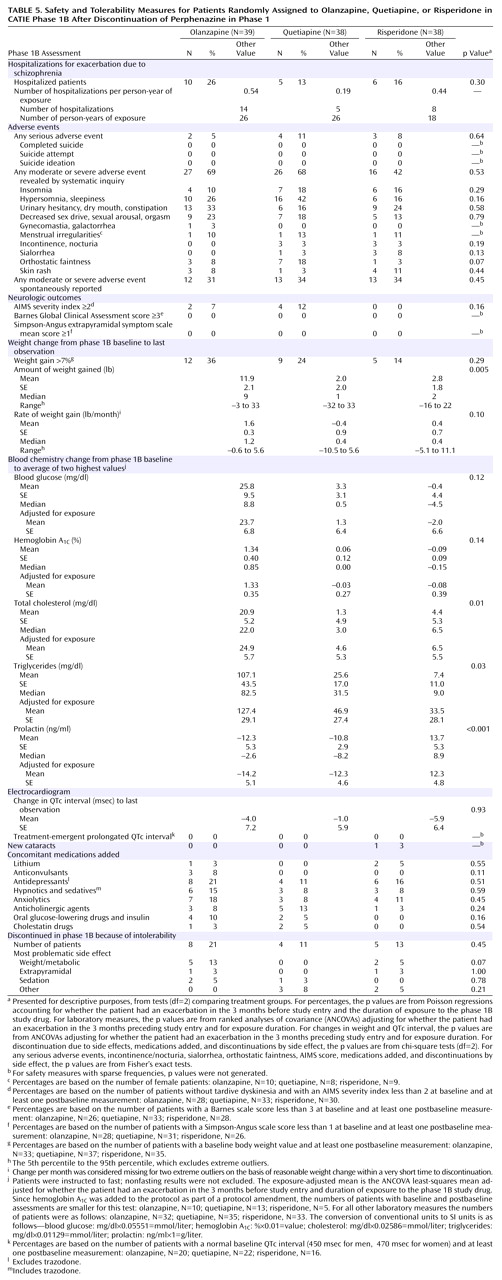
Adverse Events and Safety Outcomes
The rates of adverse events and side effects are listed in
Table 5 . Accounting for multiple hospitalizations and for the differential time in treatment, we found that hospitalizations for exacerbation of schizophrenia in the quetiapine group occurred 0.19 times per person-year of treatment, as compared to 0.44 for risperidone and 0.54 for olanzapine.
There were no substantial differences between the drugs in the adverse events reported in
Table 5 other than weight and metabolic changes, which will be described below. There was no difference between the drugs in the incidence of serious adverse events. There were no completed suicides or suicide attempts reported in this phase of the study.
There were no differences among the drugs in the incidence of extrapyramidal side effects, akathisia, or abnormal movements, as reflected by rating scale measures of severity or the reasons for discontinuing treatment.
The patients assigned to olanzapine gained more weight than the patients who took the other drugs, with a mean gain of 1.6 pounds per month. A weight gain of more than 7% of the baseline body weight occurred in a larger proportion of patients taking olanzapine than patients taking risperidone or quetiapine. No patients assigned to quetiapine discontinued treatment because of weight gain or metabolic side effects, whereas the rate for patients taking risperidone was 5% and for olanzapine it was 13%.
Olanzapine was associated with substantial increases in total cholesterol and triglyceride levels, while risperidone and quetiapine were associated with more modest increases in these measures, even after the duration of drug treatment was controlled for (
Table 5 ). Only risperidone was associated with a substantial increase in prolactin level.
There were no substantially different effects of the medications on the electrocardiographic QTc interval or the incidence of new cataracts.
There were also no substantial differences among the study drugs in concomitant medications added during the phase.
Discussion
In this group of patients with chronic schizophrenia who were randomly assigned to a new antipsychotic after discontinuing the first-generation antipsychotic perphenazine, quetiapine and olanzapine were more effective than risperidone, as reflected by longer time to all-cause discontinuation. The results of this study are somewhat different from the previously reported results of phases 1 and 2 of the CATIE study
(1 –
3) . In phase 1, which involved 1,014 individuals assigned to one of these three drugs, the effectiveness of risperidone and quetiapine was similar, and both were less effective than olanzapine
(1) . Among the 50 participants in phase 2E who were assigned to one of these three drugs, there were no significant differences in overall effectiveness
(2) . In phase 2T, which involved 307 individuals assigned to one of these drugs, the effectiveness of risperidone and olanzapine was similar and was greater than that for quetiapine
(3) . Among the 114 participants in phase 1B, quetiapine was more effective than in the previous reports, and risperidone was less effective. Olanzapine was again quite effective relative to other treatments, in this case similar to quetiapine but more effective than risperidone.
Thus, there are considerable individual differences in response to antipsychotic treatment that likely depend on as yet unknown patient characteristics. It may be that the patients who entered phase 1B were different in some clinically important way from those who had entered phase 1. For example, although the phase 1B patients were very similar to those entering phase 1 of the study with regard to age, duration of illness, and symptom severity, all patients had received and not responded well to (for one reason or another) perphenazine. They may have represented a group of patients who are relatively unresponsive to or intolerant of the higher affinity for the dopamine D
2 receptor that is characteristic of older antipsychotic drugs. In this context, quetiapine is the least and risperidone the most like perphenazine, with olanzapine being intermediate
(9) . However, because the older drugs are not homogeneous, the results of this study must be applied with caution to situations in which patients are discontinuing other older antipsychotics.
We explored the possibility that quetiapine performed relatively well compared to risperidone in this phase of the study because of similarities in the side effect profiles of risperidone and perphenazine
(1) and that those who had discontinued perphenazine in the preceding phase might respond better to quetiapine because its side effect profile (particularly with respect to extrapyramidal symptoms and hyperprolactinemia) is least like that for perphenazine. In particular, we expected that patients who discontinued perphenazine because of intolerability would also not tolerate risperidone very well. We found some evidence to support this in that among the 37 patients who had discontinued perphenazine because of intolerability, none stopped taking quetiapine because of intolerability, compared to a majority of those taking olanzapine or risperidone.
The doses of the medications used in this phase may also have affected outcomes. The doses in this phase are similar to those in previously reported phases of the study that did not specifically target patients with poor symptom response (i.e., phases 1, 1A, and 2T but not phase 2E), but the mean modal dose of risperidone (3.7 mg) was lower than in phase 1/1A (3.9 mg) and phase 2T (4.1 mg). Because a large proportion of the individuals assigned to risperidone discontinued treatment because of patient decision (32% of subjects assigned to risperidone compared to 16% for olanzapine and 13% for quetiapine), fewer people assigned to risperidone may have achieved an optimal dose than in previously reported phases of the study. The relatively poor effectiveness of risperidone in phase 1B may reflect this relatively low mean modal dose. The mean modal dose of quetiapine (586.1 mg) was somewhat higher than in phases 1/1A (543.4) and phase 2T (565.2), while the mean modal doses for olanzapine were similar across these phases (phase 1B: 20.7 mg/day, phase 1/1A: 20.1, and phase 2T: 20.5). Given that dose titration was based on the clinicians’ judgments, these results suggest that the doses achieved reflect the response characteristics of the patients (i.e., patients less tolerant of adverse effects received lower medication doses, and patients less responsive in terms of symptom reduction received higher doses). The results of phases 1 and 2 are consistent with this reasoning.
The main outcome measure in the CATIE study, all-cause treatment discontinuation, is a global measure of a drug’s acceptability and effectiveness. By this measure, none of the study drugs was highly effective, in that only 16%–42% of the individuals continued to take any drug for the duration of the trial. It is important to note, however, that discontinuation of a drug does not mean that treatment has failed. Multiple drug trials are commonly needed before one is found that is adequately efficacious, safe, and acceptable for an individual. The expectation is that successful treatment with an antipsychotic will facilitate clinically meaningful improvements in quality of life and functioning.
There are several limitations to this study. This is the fourth report from CATIE of a double-blind and randomized comparison of olanzapine, quetiapine, and risperidone. The analyses reported here were exploratory. The study group size of 114 is relatively small, and although differences were detected in the primary outcome measure, there was limited power to detect treatment differences among secondary outcome measures.
The findings from this phase of CATIE can be viewed in the context of recommendations about selection of sequential treatments that are contained in widely disseminated algorithms and guidelines for treatment of schizophrenia
(10 –
12) . In each of these sets of recommendations, guidance in selecting an antipsychotic after an unsuccessful trial is limited to discussion of side effect profiles (except for the recommendation to use clozapine in instances of refractory illness). It would clearly be of benefit to clinicians and patients if more specific recommendations could be based on information from clinical trials such as this one or from administrative databases that can provide “practice-based evidence”
(13) .
Quetiapine and olanzapine were more effective than risperidone in this group of patients who had just discontinued perphenazine. As in other phases of the study, olanzapine was at least as effective as comparator drugs in spite of its association with substantial weight gain and adverse effects on lipid metabolism. In this phase, quetiapine was very similar to olanzapine in overall effectiveness. The study provides more evidence to clinicians, patients, and policy makers that response to antipsychotic drug treatment is heterogeneous.
The individual variations in response to medications are likely due to multiple factors that may be difficult to elucidate. Response to a treatment is affected by patient characteristics, including an individual’s toleration of side effects. Previous treatment history and response to previous medications may be informative in selecting a new antipsychotic. Studies of how responses to all of the various antipsychotic drugs differ following failure of any individual drug may not be feasible, but the sequential randomizations of the CATIE study design allow for a determination of the effectiveness of a specific sequence of antipsychotic treatments and offer information that may help individual patients and physicians find an effective medication sooner than might otherwise be possible.