The ability to restore impaired psychosocial functioning is among the most severe challenges in schizophrenia treatment and a linchpin in the growing emphasis on achieving recovery in severe mental illness
(1) . Meaningful improvement in interpersonal relations, role performance, and community living skills may substantially lag behind symptomatic improvement. Achieving these gains is an increasingly important outcome in clinical trials in schizophrenia
(2 –
4) .
The advent of second-generation antipsychotics, with presumed superiority to first-generation antipsychotics in efficacy and tolerability, offered renewed promise of gains in “real-world” outcomes such as psychosocial functioning. This premise was based on the reduced burden of side effects such as extrapyramidal symptoms and sedation (which serve as barriers to instrumental daily activities), negative symptoms (which sap interest and initiative), and florid positive symptoms (which can cause further disorganization and social rejection). Measurement of psychosocial functioning and quality of life varies across many disease and nondisease specific instruments, making comparisons across schizophrenia studies difficult. The most common measure of psychosocial functioning employed in schizophrenia clinical trials is the Quality of Life Scale
(5), a widely used clinician-rated scale of social functioning, interpersonal relationships, vocational functioning, and psychological well-being, originally developed to measure a schizophrenic deficit syndrome.
Published studies of psychosocial functioning comparing first-generation and second-generation antipsychotics in terms of Quality of Life Scale scores have had variable outcomes
(2 –
4,
6 –
10) . Most studies evaluating Quality of Life Scale outcomes have focused on the benefits of clozapine, risperidone, and olanzapine, but many of these studies had design limitations, including small sample sizes, lack of appropriate controls, relatively short follow-up, and possible industry sponsor bias. As a result, there is equivocal evidence of superiority of one second-generation antipsychotic over another or of second-generation antipsychotics over first-generation antipsychotics in terms of psychosocial functioning, although one recent review concluded that second-generation antipsychotics were superior to older agents in functional gains
(4) .
This NIMH-sponsored study compared the effects of olanzapine, perphenazine, quetiapine, risperidone, and ziprasidone on psychosocial functioning as measured by the Quality of Life Scale in patients with chronic schizophrenia
(11 –
22) . Reports on the outcomes from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) investigation found that patients in the initial randomization (phase 1) who received olanzapine had longer time to treatment discontinuation than did those receiving some of the other drugs but had greater side effect burden related to weight gain and related metabolic sequelae
(15) . None of the other three second-generation antipsychotics showed greater efficacy or tolerability than the first-generation drug perphenazine. All of the antipsychotic treatment groups had small but significant improvements in neurocognition, but there was no difference among them after 2 months of treatment
(21) . In patients who discontinued phase 1 and chose to enter the “efficacy pathway” (phase 2E, which included clozapine), time to treatment discontinuation was significantly longer with clozapine than with quetiapine or risperidone but not with olanzapine
(17) . In the remaining patients who entered the tolerability pathway (phase 2T), time to treatment discontinuation was longer for patients treated with risperidone and olanzapine than with quetiapine and ziprasidone
(16) . Finally, a report on cost-effectiveness across CATIE treatment phases found treatment initiated with perphenazine least costly by $200–300/month, with no notable differences on average PANSS scores or quality-adjusted life year ratings between perphenazine and any of the atypical antipsychotics
(22) .
The primary hypothesis of the current study was that improvement in psychosocial functioning would be significantly different among these treatments. To maximize the opportunity for treatment to result in measurable improvements in psychosocial functioning, we reasoned that 12 months of treatment would allow time for adjunctive psychosocial and rehabilitative treatments to be effective and for new skills and competencies to develop, while providing the best test of pharmacologic benefit. Secondary objectives included changes in the Quality of Life Scale total score at months 6 and 18 and comparisons of the four Quality of Life Scale subscale scores at all time points. One consideration in selecting 12 months as the primary endpoint was the loss of patients to treatment discontinuation in phase 1: by 12 months, approximately one-third of patients remained in their initial phase 1 treatment assignment. At 6 months, a larger group (approximately 45% of patients) was evaluable but had half of the potential treatment time to make gains in psychosocial functioning. Weighing these alternatives, 12 months was selected as the primary endpoint.
Method
Study Design and Measures
The CATIE study was initiated by the National Institute of Mental Health to determine the comparative effectiveness of antipsychotic drugs. Its rationale, design, and methods have been previously described
(11 –
14), and treatment effects on discontinuation rates and symptoms have been reported
(17) . The study was conducted between January 2001 and December 2004 at 57 U.S. clinical sites (16 university clinics, 10 state mental health agencies, seven VA Medical Centers, six private nonprofit agencies, four independent practice sites, and 14 mixed system sites). Patients were randomly assigned to receive olanzapine, perphenazine, quetiapine, risperidone, or ziprasidone under double-blind conditions and were followed for up to 18 months or until treatment was discontinued for any reason (phase 1). Patients whose assigned treatment was discontinued could receive other treatments in phases 2 and 3
(11,
16,
17) . Patients whose assigned treatment in phase 1 was discontinued could enter phase 2
(11) . If the phase 1 treatment was perphenazine, patients then received randomized, double-blind treatment with olanzapine, quetiapine, or risperidone (phase 1B). If patients in phase 1B again discontinued treatment, then they entered phase 2.
In phase 2, patients and their study doctor could choose between two randomization pathways. The “efficacy” pathway (phase 2E), which was recommended to individuals who discontinued the previous treatment due to inefficacy, compared open-label clozapine treatment to double-blind treatment with olanzapine, quetiapine, or risperidone. The “tolerability” pathway (phase 2T), which was recommended to individuals who discontinued the previous treatment due to intolerability or who declined the efficacy pathway, compared double-blind treatment with olanzapine, quetiapine, risperidone, or ziprasidone.
Participants
Eligible patients were 18 to 65 years of age who had received a diagnosis of schizophrenia, as determined with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), and who were able to take oral antipsychotic medication as determined by the study doctor. Patients were excluded if they had a diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders; had a history of serious adverse reactions to the proposed treatments; had had only one schizophrenic episode; had a history of treatment resistance, defined by persistence of severe symptoms despite adequate trials of one of the proposed treatments or prior treatment with clozapine; were pregnant or were breast-feeding; or had a serious and unstable medical condition.
The study was approved by the institutional review board at each site, and written informed consent was obtained from the patients or their legal guardians.
Interventions
Identical-appearing capsules contained olanzapine (7.5 mg), quetiapine (200 mg), risperidone (1.5 mg), perphenazine (8 mg) or, after January 2002, ziprasidone (40 mg). Patients with current tardive dyskinesia could enroll but a stratified randomization scheme prevented their assignment to treatment with perphenazine. The dose of the medications was flexible, ranging from one to four capsules daily, based upon the study doctor’s judgment. Concomitant medications were permitted throughout the trial, except for additional antipsychotic agents. Patients had monthly visits with study doctors.
Objectives and Outcomes
We hypothesized that there would be significant differences among olanzapine, perphenazine, quetiapine, risperidone, and ziprasidone in improvement in psychosocial functioning as measured by the Quality of Life Scale.
The Quality of Life Scale
(5), administered at 6, 12 and 18 months, contains 21 items with four subscales: interpersonal relations (household, friends, acquaintances, social activity, social network, social initiative, withdrawal, sociosexual relations), instrumental roles (occupational or educational role, work functioning, work level and satisfaction), intrapsychic foundations (sense of purpose, motivation, curiosity, anhedonia, aimless inactivity, empathy, emotional interaction), and common objects and activities (commonplace objects, commonplace activities). Each item is rated on a 7-point scale (0–6). A total score is calculated by taking the mean of 21 items. Each subscale is calculated by taking the mean of the subscale items. Higher scores reflect higher functioning (i.e., scores of 5, 6 reflect unimpaired functioning).
Additional outcomes included extrapyramidal symptoms
(23), scores on the Positive and Negative Syndrome Scale (PANSS), clinician-rated Clinical Global Impression status, substance use
(24), depressive symptoms
(25), attitudes toward medication
(26), self-rated health status according to the 12-item short-form questionnaire from the Medical Outcomes Study, and neurocognitive functioning
(14,
20) .
Statistical Analysis
For consistency and comparability, the statistical methods in this study were the same as those used in the original publication from the CATIE trial
(15) . The range of analytic approaches to evaluating treatment gains were constrained by the periodicity and limited number of time points at which the Quality of Life Scale was used. The primary objective was the comparison of treatment groups for the change from baseline to month 12 in Quality of Life Scale score. Secondary objectives included treatment comparisons for change in the four subscale scores at month 12; changes at months 6 and 18 are descriptive in nature. Treatment groups were compared using analysis of covariance (ANCOVA), adjusting for baseline score, whether the patient had required crisis stabilization in the 3 months preceding study entry and tardive dyskinesia status where applicable (entry into phase 1A, which excluded perphenazine). All comparisons involving perphenazine were limited to the cohort of patients without tardive dyskinesia. Because ziprasidone was added after approximately 40% of the patients had been enrolled, ziprasidone comparisons are secondary and were limited to the cohort of patients who underwent randomization after ziprasidone was added.
The change from baseline in Quality of Life Scale score was evaluated for overall statistical significance between the four primary treatment groups at month 12 relative to p=0.05 with the use of a test with three degrees of freedom, excluding patients with tardive dyskinesia (dataset I). If the overall test was significant, perphenazine was then compared with each of the other atypical antipsychotics by a Hochberg modification of the Bonferroni adjustment for multiple treatment comparisons, in which the largest p value was compared to 0.05 and the smallest p value was compared to 0.05/3=0.017. In addition, the three atypical drugs were compared to each other relative to p≤0.05 via step-down testing: pairwise comparisons were evaluated only if the p value from the 2-df test was ≤0.05 (dataset II, tardive dyskinesia patients included). The ziprasidone group was compared with perphenazine and the other three atypicals within the ziprasidone cohort using a Hochberg adjustment for four treatment comparisons, in which the smallest p value was compared relative to 0.05/4=0.0125 (dataset III, tardive dyskinesia patients excluded for comparison with perphenazine, and dataset IV, tardive dyskinesia patients included).
Statistical significance of the Quality of Life Scale subscale scores were applied in the same manner as the total score with an additional step. Overall significance for each subscale was determined by using a Hochberg adjustment for the number of domains, in which the largest p value was compared to 0.05 and the smallest p value was compared to 0.05/4=0.0125. For each subscale, further adjustment for multiple treatment comparisons was then applied to the significance level assigned in the overall stage.
Baseline and postbaseline correlates of change in Quality of Life Scale score at 12 months were identified via Pearson and Spearman correlations, t tests, and analysis of variance. Potential correlates included patient demographics, baseline antipsychotic use, substance abuse, site characteristics (type and measure of urbanicity), and baseline and change from baseline scores measuring symptoms, neurocognitive functioning, extrapyramidal symptoms, and depression, as well as outpatient service use during the 12 months and compliance with study drug. Stepwise regression was used to develop a set of predictors, each with p value of <0.05 in the final model. A sensitivity analysis of the primary treatment comparisons adjusted for these significant covariates and tested for interactions between covariates and treatment group.
Analyses of the Quality of Life Scale score at individual time points were confirmed with a single mixed model with fixed effects for baseline value, prior crisis stabilization, tardive dyskinesia status, treatment group, ziprasidone cohort, time classification (6, 12, and 18 months), and baseline-by-time and treatment-by-time interactions. End-of-phase assessments that did not occur at 6, 12, or 18 months were excluded from analyses. The correlation of the repeated measures was modeled via a random subject intercept and an unstructured covariance matrix. Results from the repeated measures model based on three time points should be viewed cautiously, since it assumes that the large amount of missing data caused by phase discontinuations is missing at random and produces estimates based on the correlation structure of the nonmissing data.
RESULTS
Characteristics and Dispositions of Patients
The enrollment, allocation, and follow-up of study patients were described previously
(15 –
17) . Fourteen hundred ninety-three patients were enrolled in the study and randomly assigned to a treatment condition. Data from 33 subjects at one site were excluded prior to analysis for poor quality. The 455 patients who completed the Quality of Life Scale at baseline and were available at the primary endpoint (12 months postbaseline) are the primary cohort for this report. With the exception of a few missing Quality of Life Scale assessments, this group represents the one-third of patients in phase 1 of the study who had not discontinued the original medication assignment by 12 months. The baseline demographic and clinical characteristics of this retained cohort as well as those who completed the Quality of Life Scale at baseline but were unavailable at 12 months (N=985) are described in supplemental Table A that accompanies the online version of this article. The group still available for assessment at 12 months were older, had less severe psychopathology as reflected in lower PANSS total scores, were more favorable toward antipsychotics as reflected in higher Drug Attitudes Inventory scores
(26), had fewer depressive symptoms as rated on the Calgary Depression Scale
(25), were less likely to use or abuse alcohol and/or illicit drugs, were more likely to have been receiving risperidone prior to randomization and less likely to have been receiving no antipsychotics. The Quality of Life Scale total score and the subscales of interpersonal relations and instrumental roles were also higher, indicating higher baseline psychosocial functioning.
Patients available for Quality of Life Scale assessment at 12 months had far longer time to discontinuation duration in phase 1 than those unavailable at 12 months (17.6 versus 3.8 months) and had higher mean rates of compliance during the phase of treatment (91.2 versus 73.8%) (data available in supplemental table B that accompanies the online version of this article). Mean modal dose in capsules was higher for those available at 12 months (2.8 versus 2.6 capsules), and a larger proportion of those available at 12 months achieved a maximum dose of four capsules (40.0% versus 30.1%).
Assessment of psychosocial functioning at baseline, 6, 12, and 18 months demonstrated the level of patient attrition: 45% remained at 6 months, approximately one-third remained at 12 months, and approximately one-quarter remained at 18 months. At baseline, the highest subscale scores were seen in the common objects and activities scale, which reflects engagement in mainstream vocational, recreational, or instrumental task-related activities. Next highest scores were seen in the intrapsychic functioning scale reflecting a sense of purpose, motivation, and curiosity followed by the instrumental relations reflecting social interactions and initiative. Lowest scores were found in instrumental roles scale, which reflects occupational and educational functioning. All scores were consistent with a moderately impaired and vocationally disabled chronic schizophrenia population. Largest increases in psychosocial functioning were seen between baseline and 6 months, with more modest incremental gains at 12 and 18 months. By 18 months, retained patient total scores increased 0.41 from baseline, reflecting a small effect size. Largest gains were seen in the instrumental roles and common objects and activities subscales (data presented in supplemental Table C which accompanies the online version of this article).
In order to evaluate improvements in functioning from baseline, patients retained at 12 months were stratified by baseline Quality of Life Scale scores (data not shown). Patients with a baseline score of less than 2.0 (23% of the sample) made the largest gains (mean=0.71 [SD=0.96]; p<0.01). Those with scores from 2.0 to less than 3.0 (34% of the sample) made more modest gains (mean=0.32 [SD=0.97]; p<0.01), while those 3.0 or above (43% of the sample) on average made no gains and declined somewhat.
Effects of Treatment on Psychosocial Functioning
Changes after 12 months
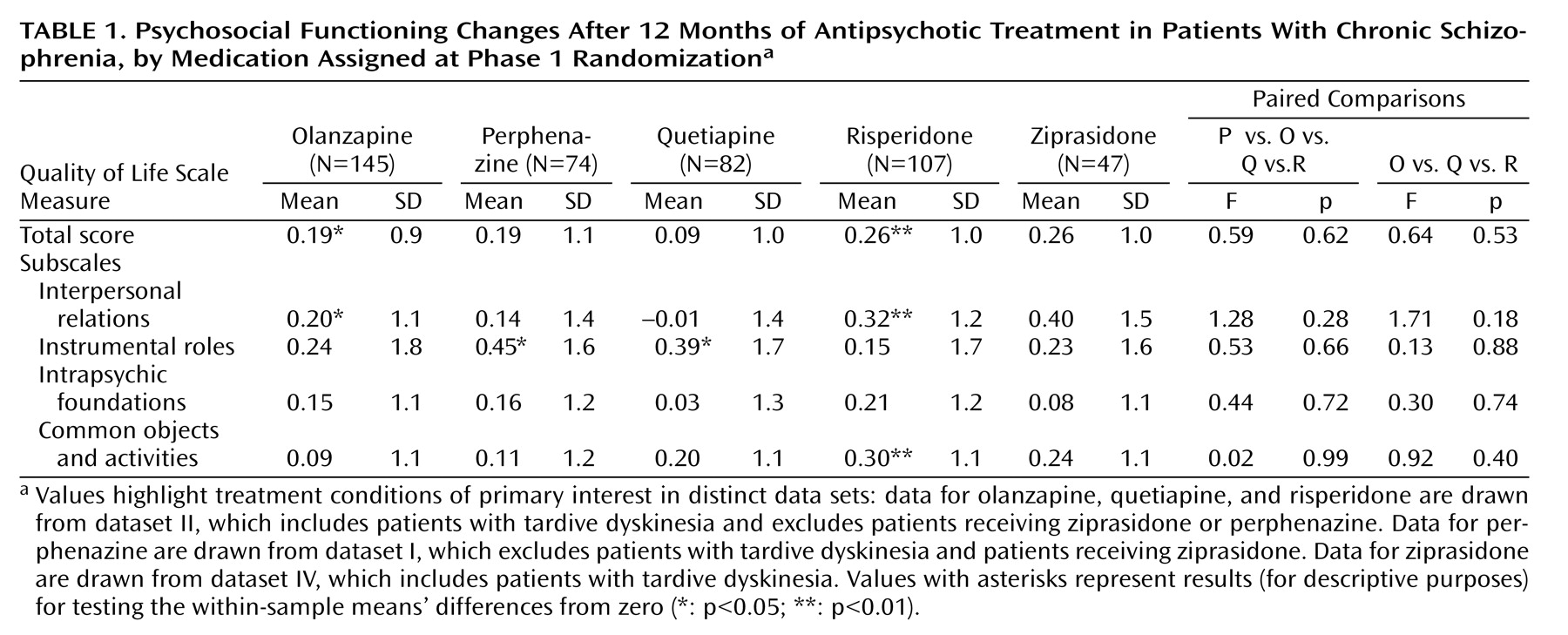
Psychosocial functioning change, as measured by change in the Quality of Life Scale total score from baseline to 12 months, adjusted for baseline score and whether the patient had required hospitalization or crisis stabilization in the 3 months prior to study entry, was the primary outcome measure in this study. This measure improved at the level of p<0.05 or greater within the groups that included patients with tardive dyskinesia (dataset II) for the olanzapine (0.19) and risperidone (0.26) treatment groups (see supplemental Table D, which accompanies the online version of this article). Improvement was comparable for the perphenazine (0.19) (dataset I) and the ziprasidone treatment group (0.26) (dataset IV) but not for the quetiapine (0.09) (dataset II) treatment groups. Smaller sample sizes for the perphenazine and ziprasidone groups likely reduced the power to demonstrate differences at the level of p<0.05. Several subscale improvements at the level of p<0.05 or greater can be seen. However, as seen in
Table 1, there were no overall significant differences among the treatment groups in the amount of change in the Quality of Life Scale total score or subscale scores at 12 months.
Changes after 6 months
Quality of Life Scale score changes at 6 and 18 months were also examined as secondary outcomes (data not shown). Psychosocial functioning change, as measured by change in the Quality of Life Scale total score from baseline to 6 months, was improved at the level of p<0.05 or greater for the olanzapine (0.26), risperidone (0.21), and quetiapine (0.16) treatment groups in the analyses that included patients with tardive dyskinesia (dataset II). In addition, perphenazine statistically approached this level of improvement (0.17 [p=0.055]) in the analyses that excluded tardive dyskinesia patients (dataset I). This level of improvement was also seen with ziprasidone (0.27) (dataset IV). However, there were no significant differences among the treatment groups in the amount of change in the Quality of Life Scale total score or subscale scores at 6 months.
Changes after 18 months
Psychosocial functioning change was improved at the level of p<0.05 or greater for the olanzapine (0.35), quetiapine (0.52), and risperidone (0.42) treatment groups in the analyses that included patients with tardive dyskinesia (dataset II); no significant improvement was seen for perphenazine (0.14) (dataset I) or ziprasidone (0.11) (dataset IV). There were no significant differences among the treatment groups in the amount of change in the Quality of Life Scale total score or subscale scores at 18 months. Mixed model analyses encompassing the month 6, 12, and 18 time points also confirmed these findings.
Changes in other psychosocial functioning indicators at 12 months
In order to evaluate other potential indicators of improvement in psychosocial functioning, change from baseline to 12 months was examined by residential status, employment, instrumental activities of daily living, and leisure activities (data not shown). At baseline, 79% of the entire cohort was living independently. Of patients retained in phase 1 at 12 months, 88.7% had no change in residential status from baseline to 12 months, 6.9% had some improvement, and 4.4% had some decline. Similarly few changes were seen in employment or instrumental, social, or leisure activities. There were no differences by treatment group in change from baseline for any of these indicators of psychosocial functioning at 12 months.
Correlations between psychosocial functioning change and other measures
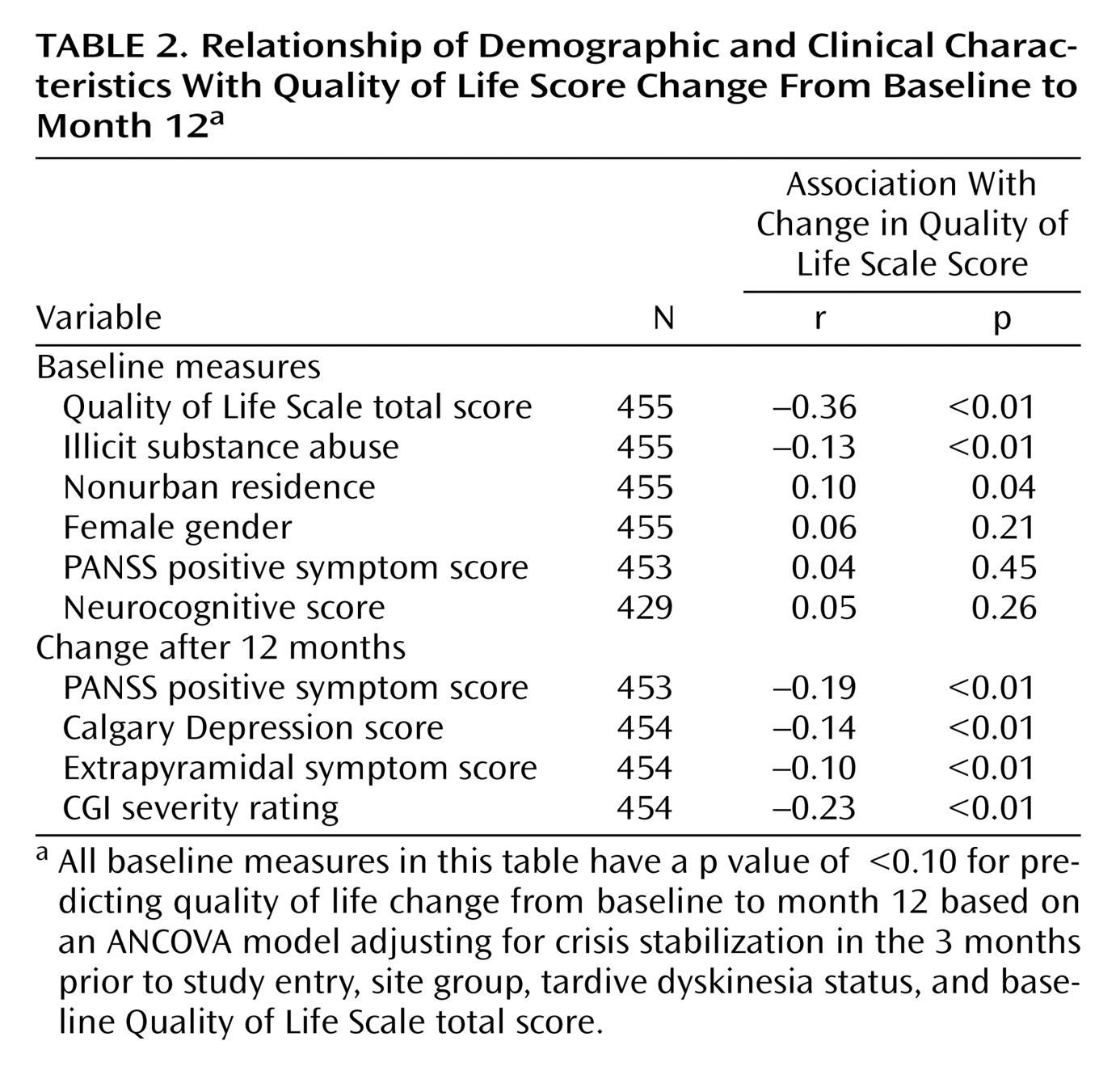
Correlations between change in Quality of Life Scale total scores from baseline to 12 months and selected baseline demographic and clinical factors are shown in
Table 2 . Among baseline measures, higher Quality of Life Scale scores at baseline were correlated with less improvement. Use or abuse of illicit substances was correlated with less improvement, and nonurban residence was correlated with greater improvement. Improvement was unrelated to use of adjunctive medications such as antidepressants or anxiolytics. Among postbaseline measures, improvement in positive symptom, depressive symptom, and extrapyramidal symptom severity scores as well as Clinical Global Impression severity ratings were correlated with more Quality of Life Scale score improvement. Due to overlap between Quality of Life Scale domains and the PANSS negative and general psychopathology scales, the total PANSS, negative symptom, and general psychopathology scores were not evaluated.
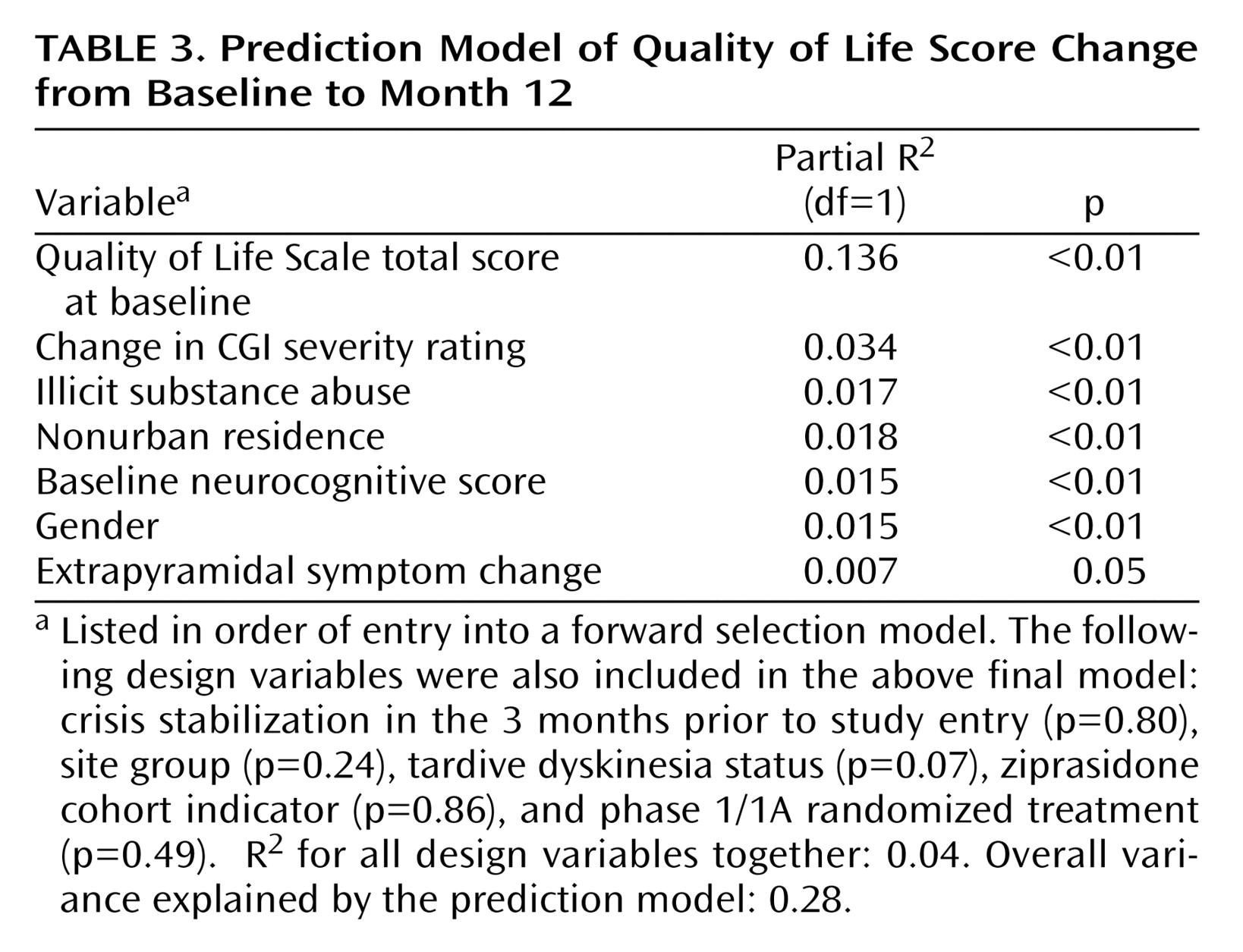
Multivariable regression modeling was used to identify a subset of unique predictors of change in psychosocial functioning from those listed in
Table 2 . The model included controls for clinical exacerbation at baseline, trial site group, treatment group, whether the patient had tardive dyskinesia, and whether the patient entered the trial after the introduction of ziprasidone. None of these adjustments were significant at the level of p<0.05 (
Table 3 ). By far the strongest positive predictor of improvement was lower baseline Quality of Life Scale score, which explained 13% of the total variation in the Quality of Life Scale change scores. Other significant positive predictors of improvement include female gender, abstinence from illicit substances, nonurban residence, higher baseline neurocognitive functioning, improvement in extrapyramidal symptoms, and improvement in CGI severity rating. Together these parameters explain an additional 11% of the total variation in the Quality of Life Scale change scores.
Changes from baseline to 12 months in self-rated physical and mental well-being as measured with the 12-item short-form questionnaire from the Medical Outcomes Study were also examined as a method to confirm these findings using self, and not clinician, report of functioning (analyses not shown). Improvements in the self-rated physical and mental well-being measures were correlated with Quality of Life Scale gains (r=0.10, p<0.05 and r=0.47, p<0.01, respectively). However, consistent with the Quality of Life Scale results, there were no differences between treatment groups at 12 months or the secondary time points of 6 and 18 months.
Changes among patients in the efficacy pathway (phase 2E)
Of the 144 patients who chose to enter the efficacy pathway, the primary analysis was restricted to the relatively small number of patients with an available Quality of Life Scale assessment at approximately 12 months of treatment (N=24) and secondarily at 6 months (N=50) (analyses not shown). Comparisons were made between clozapine-treated patients versus all others including olanzapine, quetiapine, and risperidone; these groups were found to be demographically and clinically comparable in a previous report
(17) . However, because the clozapine group had lower baseline Quality of Life Scale scores, comparisons with the other combined treatment groups were adjusted for baseline differences. There were no significant differences between clozapine and other groups in Quality of Life Scale change scores at 6 and 12 months and Quality of Life Scale changes for clozapine were comparable to those for olanzapine and risperidone in phase 1.
Changes among patients in the tolerability pathway (phase 2T)
Of the 444 patients who entered the tolerability pathway, the primary analysis was restricted to the relatively small number of patients randomized after ziprasidone had been added to the study with an available Quality of Life Scale assessment at approximately 12 months of treatment (N=67) and secondarily at 6 months (N=151) (analyses not shown). There were no significant differences between treatment groups in Quality of Life Scale change scores at 6 and 12 months, and changes were comparable to those in phase 1.
Correlations between psychosocial functioning and quality-adjusted life year (QALY) ratings
The Quality of Life Scale measure and QALY ratings utilized for the evaluation of cost-effectiveness were distinct measures. The Quality of Life Scale was based on clinician assessment of psychosocial functioning, whereas the QALY measure was a composite score based on PANSS psychopathology scores and side effect ratings
(22) . At 12 months, the QALY ratings and the Quality of Life Scale total score were moderately correlated (r= 0.38, p<0.001) as were change from baseline in Quality of Life Scale score and QALY ratings at 12 months (r= 0.32, p<0.001).
Discussion
These analyses from the CATIE study sought to examine the extent of gains in community functioning that could be expected if chronic schizophrenia patients continued with their initial randomized medication regimen in phase 1 of the study. We reasoned that the best test of expected gains would come from a consistent period of optimal drug treatment even though treatment discontinuation would limit the statistical power to detect gains. As a result, we examined change in Quality of Life Scale scores at 12 months as a primary outcome and at 6 and 18 months as secondary outcomes. Based on limited previous studies
(2 –
4,
6 –
10), we hypothesized that there would be differences in community functioning across antipsychotic medications.
While small improvements were evident across treatment groups, we found no significant differences between treatment groups and no distinct superiority of any antipsychotics in improving psychosocial functioning, consistent with recent findings from Jones et al.
(27) . Magnitude of improvement from baseline, based on within-group comparisons, were largely comparable at 12 months for the olanzapine (0.19), risperidone (0.26), perphenazine (0.19) and ziprasidone (0.26) groups at 12 months, somewhat less for quetiapine (0.09), although quetiapine treatment group gains were more comparable at 6 and 18 months.
In multivariate models of improved functioning, several predictors of improvement were notable, although their effects were small. Women were more likely to improve, consistent with studies demonstrating higher functioning in women with schizophrenia
(28,
29), which suggests that women may have relative advantage in making functional gains, perhaps because they are less likely than men to exhibit behavior that strains social supports
(29,
30) . Patients involved with illicit drugs did far worse, possibly because they were less compliant but also because they were engaged with drug-using peer networks
(18,
19) . Related to this, the finding that patients from nonurban communities made greater gains also argues for the role of adverse urban environments. Reduction of extrapyramidal symptoms was also associated with Quality of Life Scale gains, suggesting they otherwise serve as significant barriers to community functioning. Patients with higher baseline neurocognitive functioning made greater Quality of Life Scale gains, consistent with findings that better neurocognitive functioning improves treatment outcomes
(21) . However, the relatively small neurocognitive changes seen at 2 months were not strongly associated with Quality of Life Scale gains at 12 months
(21), reinforcing the idea that gains in neurocognitive functioning are instrumental to improvements in psychosocial functioning
(31,
32) .
Small sample size in the efficacy pathway and somewhat lower baseline Quality of Life Scale scores in the clozapine group limited statistical evaluation of gains in psychosocial functioning with clozapine. Once their scores were adjusted for baseline scores, improvements were comparable to the modest improvements for olanzapine and risperidone in phase 1. A larger sample would be needed to evaluate the comparative effectiveness of clozapine in improving psychosocial functioning.
Unfortunately, many phase 1 patients discontinued their initial treatment due to inefficacy, intolerability, or for other reasons before the earliest Quality of Life Scale assessment point of 6 months, making potential gains from sustained treatment unlikely. The lowest functioning patients at baseline who stayed with their initial treatment made the most substantial gains. However, in general, lower functioning patients were far more likely to discontinue treatment early. Phase 1 patients with the lowest baseline functioning (Quality of Life Scale score less than 2.0; 23% of the 12-month sample) made the largest gains, averaging approximately 0.7 points, equivalent to a medium to large effect size. Conversely, higher baseline functioning patients (Quality of Life Scale score of 3.0 or higher; 43% of the 12-month sample) made no score gains, due to an apparent ceiling effect
(31), although these findings are also consistent with regression to the mean. Thus, those most likely to benefit, at least statistically, more frequently discontinued initial treatment, and the more stable patients who were more likely to be retained made scant gains. Loss of the lower functioning and less stable patients may have weakened the ability to detect meaningful differences, although this is speculative in the absence of a more definitive study of lower functioning patients. An alternative analysis approach that examined the last observed Quality of Life Scale measurement before treatment discontinuation at any time point was considered, but we reasoned that these early discontinuations—usually in the first several months of treatment—would not accurately reflect sustainable “real world” gains from treatment.
It is possible the apparent ceiling effect among higher functioning patients reflects a reduction of symptom burden without the development of new community living skills, better interpersonal functioning, or vocational skills. All study patients, as a condition of the study, received a psychoeducational intervention designed to improve knowledge of the illness and treatment adherence. In addition, other outpatient, psychosocial rehabilitative, and vocational services varied according to local services availability. More intensive psychosocial rehabilitative services, including cognitive rehabilitation, may be needed to affect more substantial gains in functioning
(32 –
34) . Consistent provision of study medication, even over 12 months, may be necessary but insufficient to promote more robust gains in functioning. For patients unable to work, with limited access to vocational and other rehabilitative services
(33), even optimal medication may not be reasonably expected to improve the domains that bear on community functioning.