One of the more consistent physiological abnormalities reported in clinical depression is the hypersecretion of cortisol
(1,
2) . Generally, cortisol hypersecretion is regarded as a state marker of depression that remits with clinical improvement. However, some studies have suggested that subtle abnormalities in the hypothalmic-pituitary-adrenal (HPA) axis persist in patients at high risk of relapse
(3) . One convenient noninvasive measure of HPA axis activity is the increase in salivary cortisol that follows awakening
(4) . With this approach, we found that the morning increase in salivary cortisol was elevated not only in acutely depressed patients but also in recovered depressed patients who had been withdrawn from medication
(5,
6) .
The latter finding is of interest because it suggests that cortisol hypersecretion may persist during clinical remission and could represent a risk factor for further episodes of illness. However, it is unclear whether the increase in waking cortisol seen in recovered depressed patients might predate the onset of the depressive condition or could instead represent some kind of illness “scar,” that is, a consequence of repeated episodes of illness and their treatment
(7) . To resolve this issue, it is necessary to study people who are at risk of depression but who have not suffered clinical illness. Numerous risk factors for depression have been described, but one of the most reliable is family inheritance
(8) . It has been estimated that by young adulthood up to 40% of children of parents with a clinical mood disorder will have suffered a personal episode of depression
(9,
10) . The aim of the present study was to study waking salivary cortisol levels in young people who had a parent affected by depression but no personal history of depression themselves. We predicted that the waking salivary cortisol levels of these subjects would be greater than those of comparison subjects with no personal or family history of depression.
Results
Assayable cortisol samples for both occasions were returned by 49 FH+ subjects and 53 comparison subjects. The salivary cortisol levels of one comparison subject were grossly elevated (>100 nmol/liter) and were dropped from the analysis. Therefore, all subsequent analyses were carried out on 49 FH+ subjects (36 women and 13 men) and 52 comparison subjects (38 women and 18 men). The age of the FH+ subjects (mean=19.1 years, SD=0.9) was about 6 months greater than that of the comparison subjects (mean=18.7 years, SD=1.0), a difference of borderline statistical significance (t=1.98, df=99, p=0.05). The gender ratio of the groups did not differ (χ 2 =0.78, df=1, p=0.38).
Waking Salivary Cortisol Levels
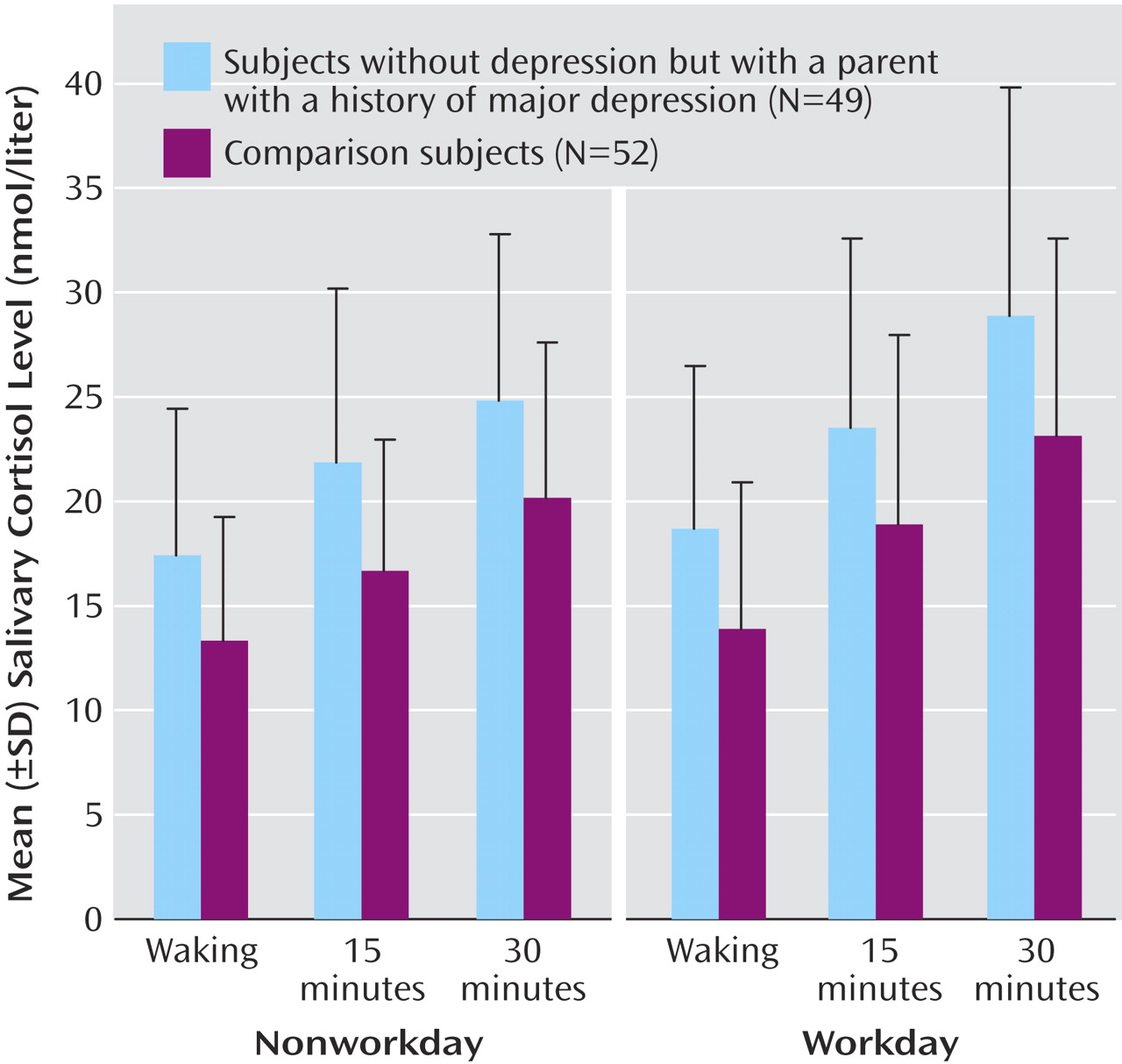
The ANOVA of the salivary cortisol data showed main effects of test day (F=10.13, df=1, 99, p=0.002), time (F=137.20, df=2, 99, p<0.001), and group (F=15.46, df=1, 99, p<0.001). There was a significant interaction between test day and time (F=4.10, df=2, 99, p<0.02), but no other significant interactions were apparent. Salivary cortisol levels rose from the point of awakening, were higher on workdays than nonworkdays, and were higher in FH+ subjects than comparison subjects at each time point (
Figure 1 ).
Significant increases in cortisol secretion of over 25% were also seen in FH+ participants when cortisol was measured as the area under the curve (workday: mean=698 nmol×minutes/liter, SD=243, versus mean=550, SD=225) (t=3.18, df=99, p=0.002) (nonworkday: mean=633, SD=216, versus mean=492, SD=166) (t=3.67, df=99, p<0.001). When we considered both groups together, the area under the curve of cortisol secretion on workdays was about 10% greater than on nonworkdays (mean=622 nmol×minutes/liter, SD=244, versus mean=561, SD=204) (t=2.97, df=100, p=0.004, paired t test). The time of awakening did not differ between the groups on either the nonworkday (FH+: mean=0846 hours, SD=1.2, versus comparison subjects: mean=0844, SD=1.2) (t=0.17, df=99, p=0.87) or the workday (FH+: mean=0749, SD=1.2, versus mean=0745, SD=1.0) (t=0.32, df=99, p=0.75).
We examined whether the cortisol area-under-the-curve in the FH+ participants was influenced by the gender of the depressed parent. For this purpose, we took the area under the curve cortisol response of each subject averaged over the two test days. Of the FH+ subjects, 33 identified depression in their mothers and 13 in their fathers (in three subjects, both the mother and the father were reported as depressed). The cortisol area under the curve in participants whose mothers had been depressed (mean=660 nmol×minutes/liter, SD=201) did not differ from those for whom fathers were depressed (mean=671, SD=219) (t=0.15, df=44, p=0.88). Both groups had significantly higher mean cortisol areas under the curve than the comparison subjects (mothers depressed: t=3.38, df=83, p=0.001; fathers depressed: t=2.65, df=63, p=0.01).
Psychosocial Data
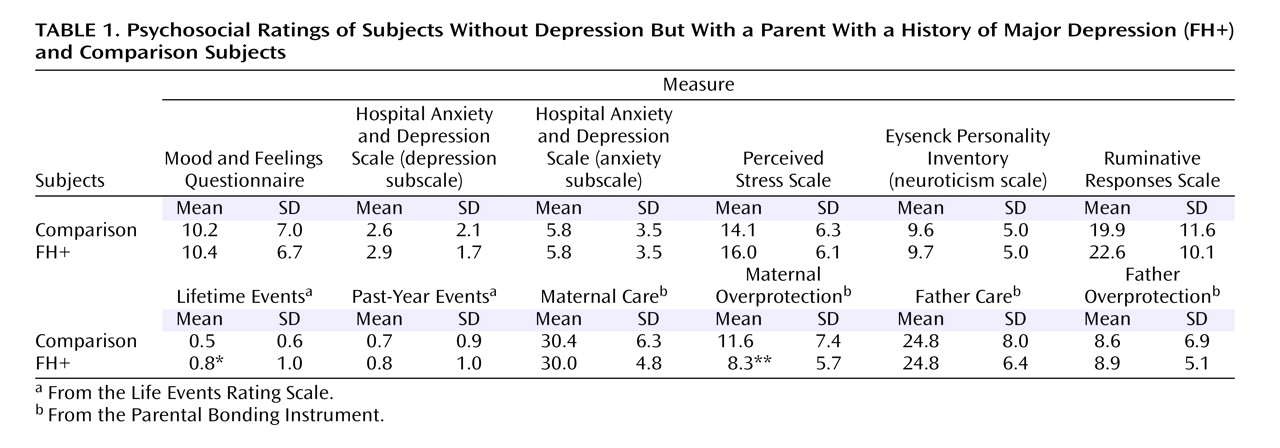
Scores for depressive symptoms, neuroticism, and perceived stress were very similar in the two subject groups (
Table 1 ). Adverse life events in the past year did not distinguish the two groups, but the FH+ subjects had a small excess of lifetime life events. Although the two groups did not differ on the father-related scores of the Parental Bonding Instrument, the comparison subjects rated their mothers as being more overprotective (
Table 1 ).
Correlations
For correlation analyses, the cortisol data for each subject were measured as the average area under the curve of the two test occasions. With this measure, there were no significant correlations between mean cortisol areas under the curve and any of the psychosocial variables shown in
Table 1, either in the FH+ subjects considered alone or in both subject groups together. In addition, there was no correlation of area-under-the-curve cortisol secretion with age or time of awakening. Some expected correlations were seen in the psychosocial data. For example, perceived stress in the past month correlated positively with the Mood and Feelings Questionnaire score (r=0.693, p<0.001), the past year’s life events (r=0.280, p=0.005), and neuroticism (r=0.590, p<0.001) and negatively with the Parental Bonding Instrument maternal care score (r=–0.33, p=0.001).
Discussion
Our findings suggest that young people at risk of depression through a positive family history have increased waking salivary cortisol levels. This increase in cortisol secretion does not appear to be explained by differences in current mental state because both FH+ subjects and comparison subjects scored very similarly on ratings of mood symptoms and perceived stress.
The increase in salivary cortisol level that follows awakening is believed to represent activation of the HPA axis
(26) . The awakening cortisol response has good intrasubject reliability, although it is modified by factors such as current perceived stress
(27) . High levels of neuroticism are associated with increased waking salivary cortisol levels, but it is difficult to distinguish the effect of trait neuroticism on cortisol secretion from the elevated levels of anxiety and depression typical of people who score highly on neuroticism scales
(28) . It has been reported previously that waking salivary cortisol is increased on workdays relative to nonworkdays, and our data support this
(29) . However, the effect of family history on waking salivary cortisol was apparent on both test days and seemed independent of the workday/nonworkday difference. We did not control for menstrual cycle status because a previous study showed no difference in waking salivary cortisol levels between the follicular and luteal phases of the cycle
(30) . In addition, the ANOVA showed no main or interactive effects of gender on waking cortisol secretion. However, in general it is advisable to control for menstrual cycle effects in neuroendocrine studies; such control might have improved the precision of our results.
What underlying mechanisms might account for the increased waking salivary cortisol level in FH+ participants? As noted above, current mental state, perceived stress, and neuroticism do not apparently play a role in this particular group of subjects. Both animal experimental and human studies indicate that childhood adversity can produce long-term modifications in the regulation of the HPA axis
(31,
32) . For example, infants whose mothers had experienced postnatal depression had higher morning salivary cortisol levels as teenagers, perhaps mediated by early attachment difficulties
(33) . However, judged by the Parental Bonding Instrument, the FH+ and comparison groups did not seem to differ substantially in their perception of childhood care, and in fact, the comparison subjects reported more maternal overprotection, although this did not correlate with cortisol secretion. Similarly, although the FH+ subjects reported a small excess of lifetime adverse events, this did not apparently account for their greater cortisol secretion. However, our life events interview did not probe specifically for childhood sexual abuse, which has been linked to the development of abnormal HPA axis function in adulthood
(32) .
It seems likely that part of the increased risk for depression in children with a depressed parent is transmitted by genetic factors
(8), and there is good evidence that genetic influences are involved in various aspects of HPA axis regulation, including waking salivary cortisol levels
(27,
34,
35) . Therefore, it seems likely that genetic differences play a part in the increased salivary cortisol secretion we observed in FH+ subjects. The increase in salivary cortisol level that follows awakening is believed to be caused by enhanced release of adrenocorticotropic hormone (ACTH) from the pituitary gland
(26), and there are several points in the HPA axis at which genetic variation could alter this process
(35) . For example, hypersecretion of cortisol in depressed patients has been attributed in part to deficient feedback by glucocorticoid receptors, leading to HPA axis disinhibition
(1, 2, 36). Glucocorticoid receptors have a number of polymorphic variants, some of which may influence cortisol secretion
(37) and could therefore be implicated in the increased waking salivary cortisol seen in FH+ subjects.
In the present group of FH+ subjects, increased cortisol secretion was not associated with clinical symptoms. However, an elevated waking cortisol level might be part of an endophenotype predisposing a subject to the development of depression in the context of life events and difficulties. Prospective studies will be required to test this proposal. If this is the case, waking salivary cortisol levels might provide a useful surrogate marker on which to test psychological or nutritional strategies designed to lower the incidence of depression in those at risk.
It is also possible that FH+ subjects could experience medical consequences of persistent cortisol hypersecretion, including, for example, increased risks of obesity and cardiovascular disease, illnesses that are known to be associated with recurrent depression
(38) . There is also much current interest in the deleterious effects of elevated cortisol secretion on synaptic plasticity and neurogenesis in the brain
(39) . For example, it has been proposed that cognitive impairments seen in depressed patients might be secondary to chronic cortisol hypersecretion affecting the cellular integrity of the hippocampus
(40) . Whether in at-risk individuals hypersecretion of cortisol in the absence of clinical depression might also result in cognitive impairment will need to be assessed in specific neuropsychological studies, perhaps in conjunction with structural imaging of the hippocampus.
A number of limitations of our study must be acknowledged. We did not systematically conduct personal psychiatric interviews with relatives for either FH+ or comparison groups; therefore, it is possible that some of the parents in the FH+ group did not suffer from depression or that some parents in the comparison group did. Presumably, however, misclassifications of this kind would tend to decrease rather than increase differences in cortisol secretion between the two groups. Also, although an increased waking salivary cortisol level might be a useful marker of vulnerability to depression, it is not clear how far an increase in cortisol secretion at one point in the day can be regarded as clinically significant, with potential pathological consequences. It is also worth noting that altered secretion of the adrenal steroid dehydroepiandrosterone (DHEA) may be a risk factor for depression in young people
(41) and that the ratio of salivary cortisol to DHEA might provide a more sensitive measure of “functional” hypercortisolemia than a measurement of cortisol alone
(42) . Further studies will be needed to assess how far increased waking cortisol secretion is a personal risk factor for subsequent depression and whether it has consequences for health and cognitive performance in the absence of depressive symptoms.