Betel nut (
Areca catechu ) is ubiquitously chewed by millions of people from East Africa to the Pacific and is the fourth most widely used drug worldwide after caffeine, nicotine, and alcohol
(1) . Arecoline, the principal betel alkaloid
(2), is a potent nonselective muscarinic agonist
(3,
4) . Several associations have been drawn between the pathophysiology of schizophrenia and muscarinic neurotransmission, including evidence for pathology of muscarinic receptors in people with schizophrenia
(5), evidence that muscarinic agonists are efficacious in animal behavioral models of schizophrenia
(6) and human clinical studies
(7), and evidence of neurochemical interactions between dopaminergic and muscarinic neural systems
(6) . The results from a previously conducted cross-sectional pilot study in Palau, Micronesia, are suggestive of a therapeutic relationship between consumption of betel nut and the symptoms of schizophrenia
(8,
9) . The aim of the current research was to test the pilot study findings with a longitudinal research design in the same setting.
Method
The study was carried out at the Behavioral Health Division, Belau National Hospital, between December 2002 and February 2004. Informed consent was obtained from 69 Palauan outpatients, 47 men and 22 women, with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder (with mainly schizophrenic course). The participants were enrolled ad hoc at the time of their scheduled outpatient contacts with the Behavioral Health Division.
Rating instruments were a self-report substance use screen designed for the study, the Positive and Negative Syndrome Scale (PANSS)
(10), the Medical Outcomes Study 12-item Short-Form Health Survey (version 2)
(11), the social functioning subscale of the Medical Outcomes Study Short-Form Health Survey (version 2)
(12), the Abnormal Involuntary Movement Scale
(13), and the Simpson-Angus Rating Scale
(14) .
The study participants were rated in three assessment cycles: an initial “baseline” assessment, then at an average of 4 months and 8 months of follow-up. The field rater was a Palauan nurse trained in the administration of the research instruments at the Clinical Research and Resource Centre, Auckland, New Zealand. The PANSS interviews of seven participants (10.8% of completers) at baseline and six participants (9.2% of completers) in the second assessment cycle were videotaped for external interrater reliability testing, carried out by a team of Clinical Research and Resource Centre clinicians. Mean interrater coefficients for the PANSS positive and negative scales in the two assessment cycles ranged from 0.75 to 0.95.
Statistical tests were between-subjects analysis of variance, with PANSS and other scale ratings as dependent variables and gender and betel-consumption groups as independent factors. Participant betel nut consumption was the average of the three assessment cycles. The allocation of the participants into chewing and nonchewing groups was an issue in that the majority of participants were betel chewers, including a large proportion of sporadic casual users. These problems were addressed by forming factor groupings around the median of consumption. Therefore, non- and low-consumption betel chewers were those below or equivalent to the median of betel consumption among users in the group under consideration, and high-consuming betel chewers were the participants above the group median.
Results
The 69 enrolled participants had a mean age of 41.9 years (SD=7.6), a mean age at onset of 22.9 years (SD=5.2), a mean age at first admission of 24.3 years (SD=9.0), and a mean duration of illness of 19.0 years (SD=7.9); the group had averaged 7.3 admissions since onset (SD=7.1). The mean chlorpromazine-equivalent dosage among the 60 participants (87.0%) receiving antipsychotic medication was 438.4 mg/day (SD=368.6). Forty-nine patients (75.4%) were receiving a mean dosage of benztropine 2.7 mg/day (SD=1.3) anticholinergic medication.
Four patients did not complete the study or were excluded because of acute phases of illness during the assessment cycles. Among the 65 completers, 49 (75.4%) were betel chewers, 63 (96.9%) smoked and/or chewed tobacco, 22 (33.8%) drank alcohol, and 29 (44.6%) smoked cannabis during the course of the study. Both men and women consumed betel and tobacco, but consumption of alcohol or cannabis was predominantly a male phenomenon: 66.7% (N=30) of the men versus 20% (N=4) of the women (χ 2 =12.09, df=1, p=0.001).
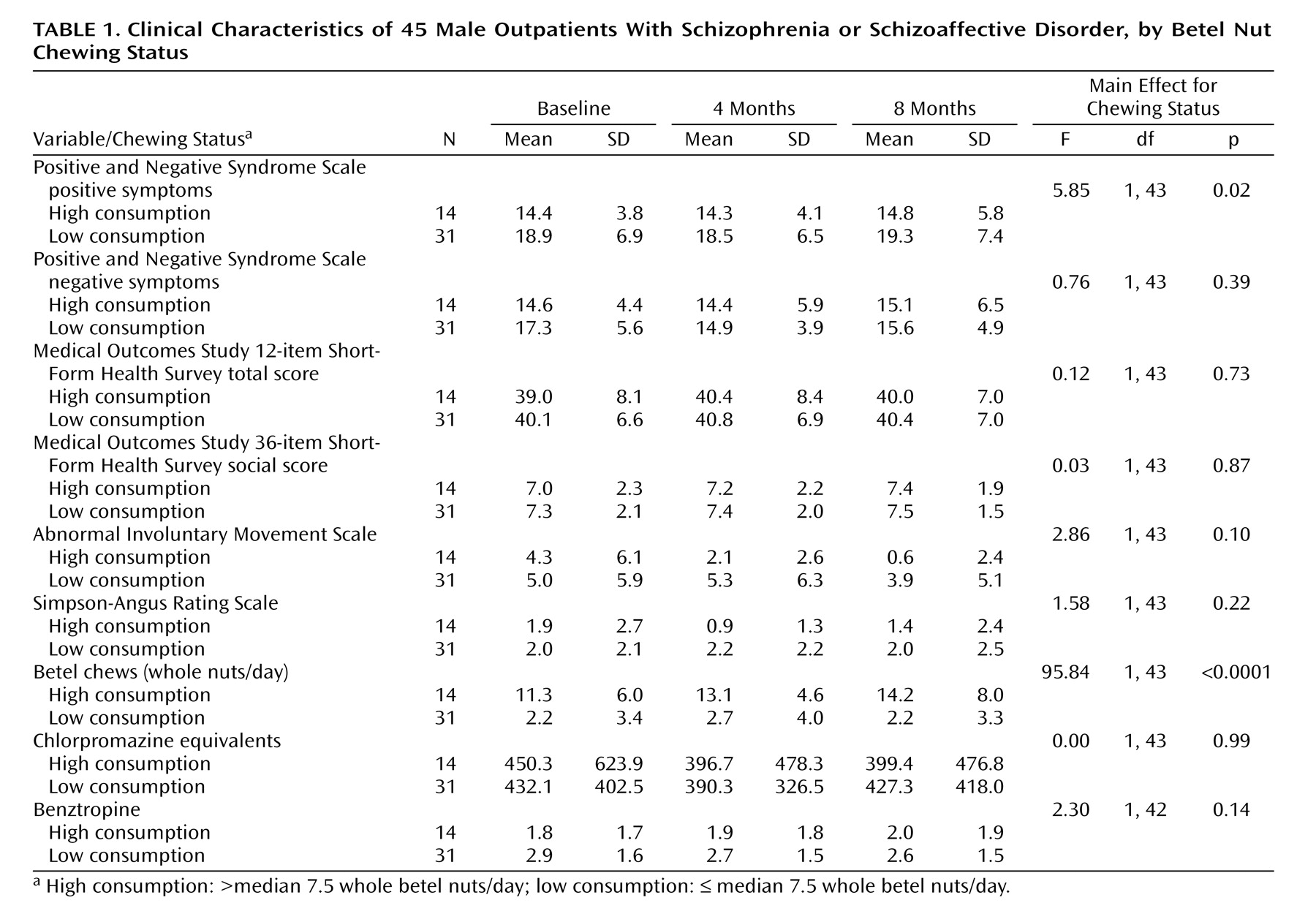
In repeated-measures analyses, there were no significant relationships between betel chewing and positive or negative symptoms for the entire cohort. However, an important finding of the 1998 pilot study was significant gender differences in symptoms and responses to betel chewing in Palauans with schizophrenia
(9) . When the data for men were analyzed separately, male high-consumption betel chewers (> median 7.5 betel nuts/day) had significantly lower positive symptoms than did low-consumption or nonbetel chewers (≤ 7.5 betel nuts/day) (F=5.85, df=1, 43, p=0.02) (
Table 1 ). In comparisons between the male high-consumption and low-consumption groups, there were no significant differences in PANSS-rated negative symptoms, global health and well-being as measured by the Medical Outcomes Study 12-item Short-Form Health Survey, social functioning as measured by the 36-item Short-Form Health Survey, tardive dyskinesia as rated by the Abnormal Involuntary Movement Scale, and parkinsonism as rated by the Simpson-Angus Rating Scale; there were also no significant differences in dosages of antipsychotic or anticholinergic medication (
Table 1 ).
In analyses between betel nut consumption and the use of other drugs, there were no relationships between male betel chewing and cannabis or alcohol use, but there was significantly reduced total tobacco consumption among the high-betel-chewing group (F=7.05, df=1, 42, p<0.02). When other drugs were assessed as independent covariates in relation to male positive symptoms and betel consumption, there were no significant interactions with mean total tobacco consumption (F=1.79, df=1, 42, p=0.19), mean cannabis consumption (F=0.87, df=1, 42, p=0.36), or mean alcohol consumption (F=0.55, df=1, 42, p=0.46).
There were no significant relationships between betel chewing and positive or negative symptoms among the female participants. However, when we compared the women to the men as a group, the women showed a tendency toward lower PANSS-rated positive symptoms (F=3.47, df=1, 63, p<0.07), significantly greater consumption of betel nut (F=8.20, df=1, 63, p=0.006), and significantly reduced total consumption of tobacco (F=7.95, df=1, 63, p=0.006).
Discussion
The results of this prospective naturalistic study are suggestive of a therapeutic relationship between betel chewing and positive schizophrenia symptoms among men, confirming similar findings in the 1998 pilot study (
8 ; see reference
9 for gender differences). Tandon and Greden (
15 ; see also reference
16 ) emphasize the importance of a modulating relationship between dopaminergic and cholinergic neurotransmission in the neurophysiology of schizophrenia, providing one possible theoretical rationale for the current study findings. They propose that muscarinic agonists exert a therapeutic effect by increasing cholinergic activity while dampening dopaminergic activity. The amelioration of positive symptoms among male high-consumption betel chewers in the current study is somewhat consistent with this model, although predicted concomitant acute extrapyramidal symptoms and elevated negative symptoms are absent. A second perspective is provided by Bymaster and colleagues
(6), who noted that “many of the neurochemical effects of muscarinic agonists such as neurotransmitter release and Fos expression are similar to those of atypical antipsychotic agents.” In this view, there are as yet poorly understood similarities between the action of muscarinic agonists and atypical antipsychotics that may underlie parallels in antipsychotic efficacy.
The absence of a relationship between movement disorders and betel consumption is counterintuitive given the cholinergic action of betel nut and may reflect tolerance to the cholinergic side effects of betel consumption among chronic users and/or documented gender and cross-cultural variability in the expression of tardive dyskinesia
(17) . The gender differences in betel nut consumption and related symptoms are also curious at first glance, but both animal
(18) and human
(19) studies have noted significant gender differences in behavioral and pharmacokinetic responses to cholinergic agents.
Previous research has suggested that betel consumption may be associated with a more favorable course of illness because chewing is a social activity pursued by relatively well individuals
(20) . Although results from the two-item social scale from the Medical Outcomes Study 36-item Short-Form Health Survey should be treated with caution, our analysis revealed no relationship between betel chewing and social functioning, or global health and well-being as measured by the 12-item Short-Form Health Survey.
There were no significant relationships between positive symptoms in men and the use of alcohol, cannabis, or tobacco in between-subjects factor analyses or when these other drugs were assessed as independent covariates. However, betel consumption is not independent of tobacco consumption in that most betel chewers in Palau also chew or smoke tobacco and because both men and women high-betel users consumed approximately half as much tobacco as non- or low-betel users. The absence of a statistical relationship between schizophrenic symptoms and tobacco consumption in the study cohort suggests that the effects of betel and tobacco use are independent, but further research in a population that does not simultaneously use tobacco and betel is recommended.
Muscarinic agents are recognized as potential treatments for cognitive deficits associated with schizophrenia and other mental disorders
(6) . Future studies should include a suitable cognitive-functioning instrument to assess the relationship between betel consumption and cognitive deficits associated with schizophrenia. To date, both of the studies investigating the relationship between betel consumption and schizophrenia have been carried out in the Republic of Palau, and further research is required to test these findings in a different physical setting and cultural context.