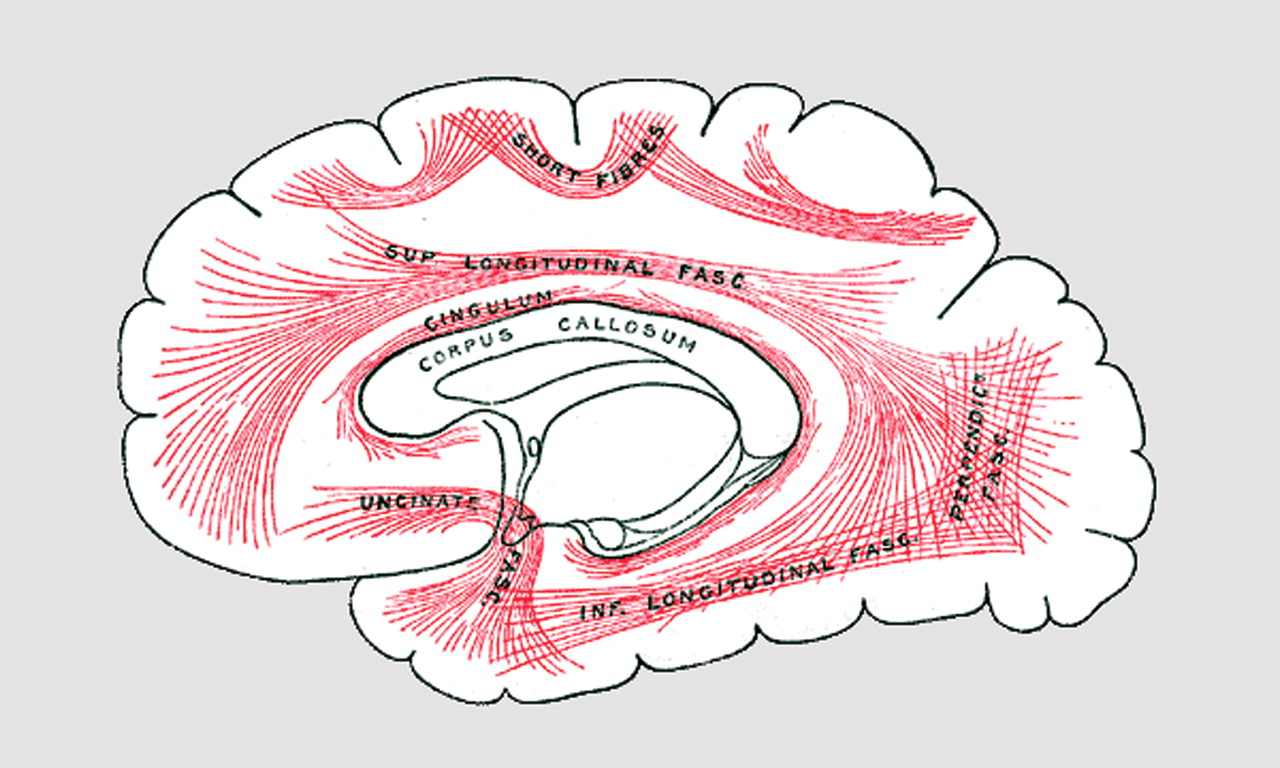
Only recently has much attention been given to white matter in schizophrenia. The primary research focus has been on gray matter, where the neuronal cell bodies, dendrites, and synapses are located, not on the white matter, which provides the wiring between cortical regions (
Figure 1 ). Structural neuroimaging studies have found volumetric abnormalities primarily in gray matter but also in white matter. Functional neuroimaging has uncovered metabolic and activation abnormalities of gray matter, but there has been no comparable technique to demonstrate the activation of white matter. However, the integrity of white matter, because of the organization of axons within bundles of myelin sheaths, can be analyzed in a different way. Diffusion tensor imaging measures the self-diffusion of water in tissue and can provide quantitative information about white matter organization. Nearly 10 years ago, the first diffusion tensor imaging study of schizophrenia was published, providing the first in vivo evidence of disorganization of white matter in schizophrenia, possibly resulting from myelin abnormalities
(1) . Animal studies have shown that diffusion tensor imaging is sensitive to changes in myelin’s integrity
(2) . Subsequent reports of reduced gene expression of myelination-related genes in frontal brain tissue
(3) and a reduced number of oligodendrocytes
(4) in subjects with schizophrenia give a plausible explanation for the in vivo diffusion tensor imaging findings. Friston et al.
(5) proposed that schizophrenia was a problem of disconnectivity, with abnormal interactions between brain regions. Since white matter is the anatomical manifestation of the brain’s connectivity, the identification of morphological and neurobiological abnormalities in white matter lends further support to the hypothesis that there is a problem in brain connectivity in schizophrenia. Three articles in this issue of the
Journal provide additional support for white matter involvement in schizophrenia.
Positron emission tomography measurement of [
18 F]fluorodeoxyglucose uptake has been used to demonstrate reduced glucose metabolism in the frontal cortex in schizophrenia but has not been used to examine white matter metabolism. Buchsbaum et al. examined relative glucose metabolic rate in both gray matter and white matter during a cognitive task in 67 unmedicated patients with schizophrenia and 103 healthy volunteers. They found a lower glucose metabolic rate in frontal gray matter as well as an increase in white matter metabolic rate in the schizophrenia patients compared with the healthy volunteers. Involved white matter regions included the corpus callosum; the internal capsule; the superior longitudinal fasciculus, which connects the anterior and posterior regions of the brain; and the uncinate fasciculus, which connects the temporal and frontal cortices. Myelination of axons permits the action potential to be transmitted more rapidly and with lower energy expenditure than in an unmyelinated axon. Thus, a finding of a white matter hypermetabolism is intriguing because it may suggest a problem in the intrinsic signal conduction or in the nature and volume of the signal. As Buchsbaum et al. suggest, there may be an inefficiency in the operation of white matter requiring greater metabolism to maintain neurotransmission, or there may be redundant or incorrect wiring requiring more extensive transmission to get a signal through. Potential cellular sources of the hypermetabolism may include oligodendrocytes, other glial cells, axons, and even misplaced neurons
(6) . The involved white matter includes major information highways between anterior and posterior parts of the brain and the frontal and temporal cortices. A technical issue with this study was that absolute glucose metabolic rates were not obtained, which would require arterial sampling. Without the absolute metabolic rates, the influence of changes in gray matter metabolism on white matter metabolism cannot be excluded. While extensive statistical analyses are provided to guard against this possibility, this interesting result will require replication with absolute metabolic rate measures. Whether medication normalizes the observed white matter hypermetabolism would also be interesting to know.
Whitford et al. examined deficits and changes in white matter volume with magnetic resonance imaging (MRI) structural brain scans. They used voxel-based morphometry to examine a cohort of 41 first-episode patients with schizophrenia and 47 healthy comparison subjects. The baseline data showed white matter volume deficits in the frontal and temporal lobes and an increase in the fronto-parietal junction. Follow-up scans at 2–3 years were available for 23 of these patients and 26 comparison subjects. The patients showed greater loss of white matter volume bilaterally in the inferior temporal cortex than the comparison subjects. In a prior publication on this cohort
(7), researchers analyzed gray matter density and found deficits in gray matter in the frontal, parietal, and temporal regions that progressed in the parietal and temporal regions over time. Controlling for gray matter volume did not change the white matter findings, which suggests that the observed white matter abnormalities were not related to gray matter abnormalities. This article thus adds to the evidence provided by Buchsbaum et al. for pathological changes in white matter that may reflect a progressive deterioration of white matter integrity.
With the advent of noninvasive in vivo neuroimaging, tissue volume abnormalities were among the first findings in schizophrenia. Analysis approaches have evolved from purely manual strategies to those with minimal user interaction. While early studies focused on specific brain regions because of the labor-intensive quantification process, the whole-brain examination approach has allowed less anatomically biased studies that provide us with a broader perspective on how much of the brain is involved. The Whitford et al. study extends this view by demonstrating baseline involvement of white matter in frontal, temporal, and parietal regions in first-episode patients and continued progression in temporal regions.
The involvement of multiple brain regions is supported by a third paper in this issue: Barch and Csernansky found bilateral deficits in activation of the prefrontal and parietal regions during working memory tasks. Working memory, the ability to maintain and manipulate information over a short time span, such as remembering a phone number long enough to dial it, has been found to be abnormal in individuals with schizophrenia. Abnormal activation of prefrontal and parietal cortex has been observed in schizophrenia patients during working memory tasks. Baddeley
(8) has proposed a model of working memory that involves short-term storage buffers and a central executive component. Barch and Csernansky sought to determine whether the abnormal activation is due to a problem in phonological coding processes located in the ventral prefrontal and parietal cortex or a defect in a hypothesized central working memory executive located in the dorsal prefrontal and parietal cortices. Fifty-seven medicated patients with schizophrenia and 120 matched healthy comparison subjects were examined with functional MRI (fMRI) while performing a verbal and a nonverbal working memory task so that the activity due to working memory as well as material type (words or images) could be analyzed. By using the appropriate comparisons, the authors were able to tease apart contributions of various brain regions to the different tasks. They conclude that a deficit in working memory function is due to a problem in the central executive component located in the dorsal frontal and parietal cortex and not due to a problem with the phonological loop. While this finding suggests that there may be a problem of functional connectivity between the prefrontal and parietal regions involved in working memory, direct examination of how the activity between the two regions is related should provide more information on the connectivity issue
(9) . Interestingly, the finding on frontal-parietal activation intersects with the white matter and gray matter abnormalities observed by Whitford et al. and the increased white matter metabolism reported by Buchsbaum et al., including in the superior longitudinal fasciculus, which connects the frontal and parietal cortices.
Future studies will need to take into account these convergent neuroimaging findings, integrating and extending them, along with other findings, in multimodality experiments with the same subject. For example, the anatomical findings from diffusion tensor imaging and morphometry need to be integrated with functional studies. Diffusion tensor imaging tractography has generated much excitement because of its impressive depictions of tracts, which are consistent with known anatomy. While the low spatial resolution of the base data and validation issues pose challenges, new analytic approaches, such as probabilistic tractography, may help mitigate some of these issues
(10) . Studies integrating fMRI and diffusion tensor imaging would be able to address the contribution that anatomical connectivity makes to observed fMRI activation abnormalities between different brain regions.
If we interpret the increased white matter metabolism as an indication that the white matter is stressed, it would be helpful to better understand the nature and causes of this stress. Proton magnetic resonance spectroscopy of white matter has found reductions in
N -acetylaspartate, generally thought to be an indication of neuronal viability
(11) . Phosphorus spectroscopy can provide even more direct information about energy stores and metabolism. Reduced myelin would directly affect neural conduction velocity and potentially affect neural synchrony
(12), which could be measured with electrophysiological techniques. Genomics is undoubtedly another important factor that will need to be integrated. A current example is the evolving story of neuregulin 1 as a susceptibility gene for schizophrenia, for which there are now connections to multiple mechanisms currently believed to be important in this illness, including the regulation of radial glia, neuronal migration, neurotransmitter receptor expression, oligodendrocyte development, and onset of puberty
(13) . Clearly, the future lies in our ability to judiciously use multimodality methods in well-designed experiments to tease apart the contributions and interactions of these various factors.
Although many of the powerful techniques described above cannot be used in human in vivo investigation of neurobiological mechanisms, the use of animal models can allow us to examine the contribution of specific molecular mechanisms to observed pathologies. A recent report
(14) described a study in which NRG1-erbB signaling was blocked in oligodendrocytes in mice, resulting in a number of the pathologies seen in schizophrenia. The mice had more oligodendrocytes but had thinner sheaths, resulting in slower electrical conduction. In addition, there was evidence that the mice had altered sensitivity to dopamine.
Future advances in schizophrenia research will depend on examinations of connections at and across multiple levels, including cellular pathways, neuronal circuitry, brain regions, and different modalities and disciplines. Each level is critical if we are to develop the understanding necessary to develop better treatments for our patients and ultimately to prevent this complex and devastating disorder.