According to the 2017–2018 National Health and Nutrition Examination Survey, 54.8% of adults in the United States take at least one dietary supplement (
1). Unfortunately, there is no worldwide unified definition or regulation of dietary supplements versus complementary medications versus prescribed pharmaceuticals (
2). Dietary supplements that are available over the counter in one country could require prescriptions in another or even be classified as controlled substances. This lack of conformity means that quality control regulations for the same product vary widely among countries, based on the classification assigned. With over 85,000 supplement products marketed and regulated in the United States under the Dietary Supplement Health and Education Act of 1994 (
2), it is practically impossible for clinicians to know about every supplement their patients might be taking. This article is a review of the drug adrafinil, sold as a purported "nootropic," or cognition-enhancing supplement, in the United States, that highlights information from credible resources for prescribers, as well as from online user reports, to learn about this loosely classified "supplement."
Pharmacology
Adrafinil (2-(diphenylmethyl)sulfinyl-
N-hydroxyacetamide) is a prodrug, which is metabolized to the active compound, modafinil, in vivo via liver metabolism (
3,
4). The R-enantiomer of modafinil has an apparent half-life of 12 to 15 hours, whereas the S-enantiomer has a much shorter half-life of 4 to 5 hours. Both undergo extensive hepatic metabolism to form inactive metabolites prior to elimination in the urine (
4). Although modafinil is regulated by the U.S. Food and Drug Administration (FDA) as a controlled substance (Schedule IV), adrafinil is sold as an unregulated supplement and can be purchased without the need for a prescription (
4). Modafinil (brand name Provigil) is FDA-approved for the treatment of excessive sleepiness associated with medical conditions such as narcolepsy, sleep apnea, and shift work sleep disorder (
5). The mechanism of action of modafinil is not fully understood; however, unlike other stimulant medications, it does not appear to exert its effects by increasing the release of monoamines. Instead, it may act at the dopamine transporter (DAT) to reduce dopamine reuptake (
4,
5). Other proposed mechanisms include modulation of glutamate and γ-aminobutyric acid (GABA) transmission, increases in serotonin release, and activation of orexin neurotransmission (
4).
Clinical Studies of Adrafinil
Animal studies for adrafinil have been reported; additionally, a few clinical trials involving human subjects, performed decades ago by French researchers, have also been reported. Michel Jouvet is considered a pioneer researcher with respect to the human use of adrafinil. In the late 1970s, he administered large doses of adrafinil to hypersomniac and narcoleptic patients, with inconsistent results (
6,
7). Subsequent studies, published in French, evaluated its efficacy in vigilance promotion and treatment of depression, in addition to its effects on motor organization, as outlined by Milgram et al (
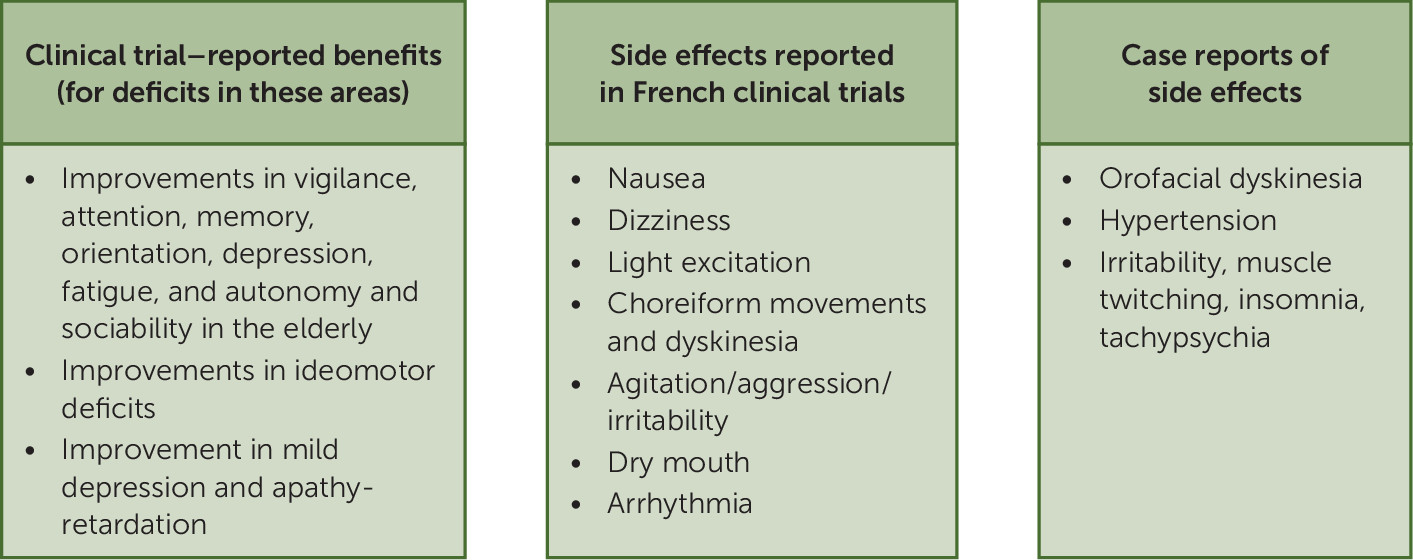
8). As described by Milgram et al., six studies were conducted that included ambulatory and hospitalized patients of minimum 45 years of age, with the majority being older than 65 years, who exhibited "problems in focusing attention, sleep, memory, and mild depression." Notably, "major psychiatric disorders" and dementia were exclusionary criteria. Overall, the results indicated improvements in vigilance, attention, memory, orientation, depression, fatigue, autonomy, and sociability in the elderly. Another French trial described by Milgram et al. reported improvements in ideomotor deficits, defined as "general suppression in voluntary movements, deficits in organizing motor sequences, paralysis of decision, and paralysis of functional motor action." A final trial reported greater amelioration of depression and psychomotor retardation, compared with clomipramine treatment, in subjects with mild depression not meeting DSM-IV criteria for major depressive disorder (
8,
9). These eight studies documented few and inconsistent side effects (
Figure 1) (
8). The review provided here has significant limitations because many study details were not reported, and thus the validity and generalizability of results are undetermined.
Adrafinil was first marketed in France in 1985 under the name adrafinil, with indications for "disorders of vigilance, attention, and ideomotor slowing in the elderly" (
10). Three case reports on the side effects of adrafinil treatment have subsequently been published in France. A 75-year-old man with atrial fibrillation, hypertension, and diabetes mellitus developed orofacial dyskinesia after 10 months of daily administration of 900 mg of adrafinil to treat daytime sleepiness (
11). This dyskinesia did not resolve even 4 months after adrafinil withdrawal and ultimately improved only after the administration of daily tetrabenazine. The dyskinesia was deemed irreversible after attempts to discontinue tetrabenazine were unsuccessful. A 63-year-old woman with a history of hypertension experienced elevated blood pressure following modafinil treatment (
12). She was successfully treated with antihypertensives and was switched to adrafinil 900 mg daily. After 2 months, her hypertension became uncontrolled again. Thus, adrafinil was discontinued, which resulted in an improvement in her blood pressure. Lastly, a 35-year-old man with depressive disorder and attention-deficit hyperactivity disorder was taking paroxetine 40 mg daily with adrafinil 900 mg daily (
13). After a year of treatment, he independently discontinued the use of paroxetine and immediately experienced irritability, muscle twitches, tachypsychia, and insomnia, which resolved when paroxetine was reintroduced. In 2011, a French marketing authorization committee reviewed the clinical studies and was unable to conclude that adrafinil provided benefit. This, in addition to safety data indicating known adverse effects, resulted in the removal of adrafinil from the French market (
10).
Systematic Review of Online Experience Reports
Although case reports and clinical trials involving adrafinil are limited, individuals have shared their personal experiences with adrafinil on the Internet. For information regarding typical use outside of a medical or research setting, various online resources were queried for experience reports, including Erowid (
Erowid.org), Bluelight (
bluelight.org), Drugs-Forum (
Drugs-Forum.com), and psychonautWiki (
psychonautwiki.org). The following data were extracted from each experience report: motivations for use; side effects noted, with special attention paid to the incidence of extrapyramidal symptoms; dose reported; how the drug was obtained; and any drugs that were co-ingested with adrafinil. The limitations of these data include reporting bias; lack of placebo controls; lack of methods to verify the dose, purity, or identity of the consumed substance; and user reports potentially arising from a biased sample of users (i.e., those who frequent online drug forums).
A total of 49 experience reports were obtained for analysis, including 43 reports (88%) from Erowid, five (10%) from Drugs-Forum, and one (2%) from psychonautWiki, as of September 29, 2020. Although adrafinil was mentioned several times on Bluelight.org, there were no discrete experience reports available for analysis.
Of the 49 experience reports obtained, 44 (90%) reported the dose ingested. The dose ingested ranged from 50 mg to 2,700 mg, with a median dose of 600 mg and mean dose of 623 mg (SD=438 mg). The source of adrafinil was referenced in 19 (38%) reports (
Table 1). Of these, 18 (95%) reported obtaining adrafinil from an online vendor, while one (5%) procured adrafinil from a friend who had purchased it from an online vendor. Adrafinil is available online through websites such as nootropicsdepot.com, purenootropics.net, zachattacksupplements.com, and newmind.com, at prices ranging from $1.87 to $4.18 per gram. No alternative names for adrafinil were identified. Each website categorized adrafinil as a "nootropic" chemical or compound.
Motivations for using adrafinil were detailed in 45 experience reports (92%) (
Table 1). The most common reasons for use were to increase focus, productivity, or motivation (59%) and wakefulness (35%). Adrafinil was also often used as a substitute for another drug, as understood from 24% of reports, due to the comparator drug being difficult to obtain, costing more, or having a worse perceived side effect burden. Most frequently, adrafinil replaced a prescription drug, such as methylphenidate, lisdexamfetamine, modafinil, or dextroamphetamine-amphetamine. In some cases, adrafinil usage was described as an alternative to caffeine intake.
The most common spontaneously reported undesired effect was malodorous urine (20% of reports) (
Table 1), followed by headache (16%), disturbed sleep (14%), anxiety or feeling "jittery" (12%), and feeling unpleasantly stimulated (10%). One individual using adrafinil for athletic performance enhancement suffered severe dehydration with significant cramping and increased body temperature. In nine cases (18%), individuals reported adverse effects from adrafinil cessation. Although withdrawal effects were variable, they included fatigue, hypersomnia, poor focus, irritability, mental exhaustion, headache, nausea, vomiting, and worsened mood.
Co-ingestion of other substances with adrafinil was referenced in 10 reports (20%). The co-ingested substances included caffeine, alcohol, cannabis, bupropion, amphetamines, suboxone, "hypertension medications," "sleeping pills," opium extract, 5-hydroxytryptophan, tryptamine psychedelics (not specifically identified), milk thistle, ginkgo, choline, kava, and oxiracetam. Caffeine and alcohol were mentioned in two reports each, and all other substances were mentioned only once.
Conclusions
Despite unclear evidence of efficacy, adrafinil has attracted many individuals seeking to utilize its proposed benefits. There is a clear risk associated with adrafinil usage, owing to the side effects that are reportedly associated with comorbidities, overuse, and cross-reactivity with other medications. These factors are exacerbated by lack of physician oversight. Here, we discussed the limited scientific evidence for adrafinil administration in the elderly, while also highlighting the personal experiences reported by individuals who used adrafinil outside of a medical or research setting. In conclusion, based on the evidence reported, we would not recommend adrafinil as a dietary supplement. Moreover, in a patient reporting adrafinil use or misuse, clinicians should evaluate for adverse effects similar to those associated with modafinil.