We reported in the last few years that smokers have brain monoamine oxidase B (MAO-B) values averaging about 40% lower than those of nonsmokers and former smokers
(1). MAO-B breaks down dopamine and also generates hydrogen peroxide, raising questions as to whether lower levels of MAO-B in smokers than in nonsmokers may contribute to smoking behaviors and epidemiology.
We report here a replication of our previous study in another group of smokers in whom we also determined whether MAO-B activity recovered after overnight cigarette abstinence.
Method
Six healthy smokers (one woman and five men; mean age=39 years, SD=13) were recruited by means of advertisement. Subjects with a history of neurologic or psychiatric disorder, head trauma with loss of consciousness, or alcohol or substance abuse (except for caffeine and nicotine) were excluded. Prescan tests ensured the absence of psychoactive drug use. The average age of the combined group of six smokers from this study and eight smokers from the previous study was 42.3 years (SD=13.4). One smoker participated in both studies. After receiving a complete description of the study, the subjects gave their written informed consent. Positron emission tomography (PET) scans were performed on a positron emission tomograph (Siemens EXACT HR+, Knoxville, Tenn.) (63 slices, 4.5 × 4.5 × 2.4 mm full width at half maximum). Each subject had two PET scans (2 hours apart) by means of [
11C]L-deprenyl-D2 (4–9 mCi, specific activity=0.2 Ci/μmol at time of injection): at baseline (mean=11.3 hours after smoking last cigarette, SD=3.2) and 10 minutes after smoking one cigarette by means of the procedure and timing described previously
(2,
3). The previous study was performed on a different scanner (Siemens CTI 931, Knoxville, Tenn.) (6.5 × 6.5 × 6.5 mm full width at half maximum)
(1).
Subjects were asked to have their last cigarette the night before the study and to refrain from smoking the morning of the study. Before the first PET scan and 10 minutes after beginning to smoke one regular cigarette (duration=5–10 minutes), subjects provided a breath sample for carbon monoxide analysis (by means of a Vitalograph, Lenexa, Kan.), and a blood sample was taken for nicotine and cotinine analysis to support their claims of overnight abstinence (nicotine levels) and for correlations of MAO-B levels and smoking dose (cotinine levels)
(4,
5).
Thirty healthy nonsmokers (mean age=41.7 years, SD=12.8) from other studies
(3,
6,
7) made up a comparison group. Sixteen were scanned on the HR+ scanner, and 14 were scanned on the CTI 931 scanner.
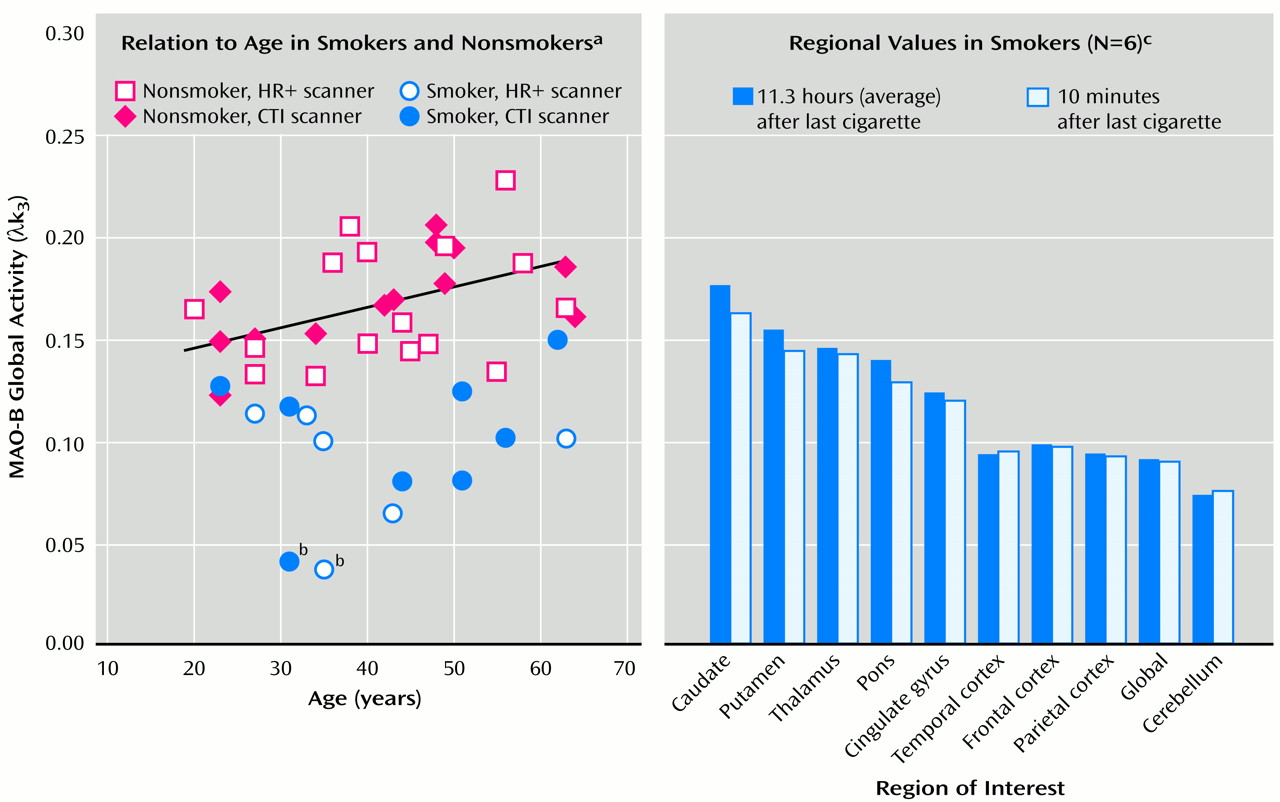
PET time-activity data from 10 brain regions, including the global region (
Figure 1), and tracer concentration in arterial plasma were used to calculate the model terms K
1 (the plasma-to-brain transfer constant that is related to blood flow) and λk
3 (a function of MAO-B activity) by means of a three-compartment model
(8), where k
3 is related to the binding of radiotracer to MAO-B in brain tissue, λ is defined as K
1/k
2, and k
2 is a term related to the efflux of tracer from the brain to the blood.
Average values for λk3 and K1 and arterial plasma concentrations for smokers and nonsmokers were compared by means of unpaired t tests, two-tailed. The degree of MAO-B inhibition in each smoker was determined by comparing individual λk3 to the nonsmokers’ mean for global value. The model terms λk3 and K1 for each brain region, the plasma nicotine and cotinine concentrations, and the expired carbon monoxide levels were compared for scan 1 (baseline) and scan 2 (after smoking) by means of a paired t test, two-tailed. Data from the two scanners were analyzed separately as well as together. Pearson product-moment correlations were performed between the percentage of MAO-B inhibition of individual smokers, plasma cotinine and nicotine concentrations, and numbers of cigarettes smoked per day.
Results
Results from the two scanners did not differ, and thus the results presented here are for the combined groups. The average global brain MAO-B level (as λk
3) was 0.17 (SD=0.03) for the 30 nonsmokers and 0.10 (SD=0.03) for the 13 smokers (t=7.50, df=41, p<0.0001) (
Figure 1). There was a higher arterial tracer concentration in smokers than in nonsmokers, although the difference did not reach significance (t=–1.90, df=39, p<0.07). The average brain MAO-B inhibition was 39% (SD=17%), with a range of 10%–77% for the 13 smoking subjects from the two studies. One of the smokers had values of 75% and 77% less MAO-B inhibition in the studies, which were 4 years apart, than nonsmoking comparison subjects. There was no significant difference in λk
3 (or K
1; data not shown) between the two scans for any brain region examined (
Figure 1). Plasma nicotine levels differed at baseline and after smoking, averaging 2.8 ng/ml (SD=2.0) and 18.3 ng/ml (SD=7.5), respectively (t=–6.37, df=5, p=0.001), whereas plasma cotinine levels did not differ. Expired carbon monoxide was generally higher in the smokers immediately after smoking than in the nonsmoking comparison subjects, but this level did not reach significance. There was no significant correlation between the percentage of brain MAO-B inhibition and plasma cotinine or nicotine concentrations or the number of cigarettes smoked per day.
Discussion
This study replicates the finding that smokers have lower brain MAO-B levels that nonsmokers
(1) and shows that brain MAO-B does not measurably recover after an 11-hour abstinence in smokers. The degree of inhibition appears to be stable over time, since repeated measures were similar in one smoker who participated in studies 4 years apart (
Figure 1).
Nicotine does not inhibit MAO-B
(9). The lack of an association between MAO-B levels and the number of cigarettes smoked per day is consistent with evidence that smoking behavior, rather than the number of cigarettes smoked, controls the dose of nicotine (and presumably other substances in cigarettes)
(4). We note that an MAO inhibitory compound was recently isolated from tobacco leaves
(10).
This study reinforces the need to report smoking status in clinical studies and to reevaluate reports that low MAO-B inhibition is a biological marker in clinical populations where the rate of smoking is high. For example, normal platelet MAO levels were recently reported in nonsmoking patients with schizophrenia
(11). Smoking remains a major public health problem; however, advances in treating smoking addiction hinge on characterizing both the neuropharmacological effects of tobacco smoke and the factors accounting for individual variability in smoking toxicity.