Although it is well established that personality traits are heritable
(1–
3), most theories of personality do not attempt to identify the specific genes involved. Cloninger and colleagues
(4), however, proposed a psychobiological model of personality that purports to map personality at the genetic level. At the psychological level, they propose that temperament and character traits can be described in terms of seven major factors. At the biological level, they argue that the temperament traits are associated with neurochemical substrates that have a genetic basis. One implication of this theory is that genes associated with neurotransmitters should be related to the hypothesized temperament traits. Another implication is that traits hypothesized to have a shared genetic basis should covary at the phenotypic level. In this study we tested both of these aspects of the temperament-character theory.
Cloninger and his colleagues assess personality with the Temperament and Character Inventory
(5), a seven-factor model with four temperament and three character dimensions. Cloninger et al. claim that the temperament dimensions of novelty seeking, harm avoidance, reward dependence, and persistence are genetically homogeneous and that two of them are associated with distinct neurochemical substrates: novelty seeking with dopamine and harm avoidance with serotonin. The model also identifies three character dimensions called self-directedness, cooperativeness, and self-transcendence, which are based on social goals and values. According to Cloninger and colleagues
(6), the psychobiological model accounts for the genetic basis of the personality phenotype, whereas alternative models of personality comprise genetically and environmentally heterogeneous factors.
Findings in molecular psychiatry presented great promise in mapping candidate genes for dimensions of human personality traits. In two studies
(7,
8) the dimension of novelty seeking was linked to the seven-repeat allele (or long form) of the 16-amino-acid polymorphism of the D
4 dopamine receptor gene (D4DR), and in another study
(9) harm avoidance was associated with the short form of a functional polymorphism (44-base-pair insertion or deletion) in the serotonin-transporter-linked promoter region (5-HTTLPR). These findings were greeted with enthusiasm because they provided empirical support for Cloninger’s claim that the temperament dimensions of novelty seeking and harm avoidance have an identifiable genetic basis. Indeed, Cloninger and colleagues themselves stated that it may be fruitful “to map genes contributing to temperament, which has a relatively simple genetic architecture and can be quantified easily and reliably by questionnaire”
(6, p. 4). Although replications have been reported
(10–
15), numerous failures to replicate the associations with D4DR
(16–
22) and 5-HTTLPR
(23–
26) raise the question of whether the temperament-character model adequately taps the genetic architecture of personality.
In the present study we examined two candidate neurotransmitter genes, D4DR and 5-HTTLPR, and Cloninger and colleagues’ measure of the personality phenotype, the Temperament and Character Inventory, in a large sample of community-dwelling adult men and women. We tested whether the novelty seeking and harm avoidance temperament dimensions of the Temperament and Character Inventory are associated with functional polymorphisms in D4DR and 5-HTTLPR, respectively. Prior studies
(7,
8) suggested that individuals with the seven-repeat allele (or long form) of D4DR score higher on novelty seeking than do people with the short form of D4DR. However, a recent study in this journal by Ekelund et al.
(21) on a cohort of almost 5,000 Finnish men and women suggested that the two- and five-repeat alleles, rather than the seven-repeat allele, were significantly associated with high scores for novelty seeking. In the current study we attempted to replicate these findings, as well as to test whether the short variant of 5-HTTLPR is associated with higher scores on the harm avoidance dimension of the Temperament and Character Inventory
(9).
Each of the dimensions of the Temperament and Character Inventory (except persistence) is assessed as the sum of scores on three to five subscales measuring more specific traits. For example, novelty seeking includes exploratory excitability, impulsiveness, extravagance, and disorderliness. If in fact these traits share a genetic basis, they should covary in the population. Factor analysis of the Temperament and Character Inventory subscales was used to test the hypothesis that temperament and character traits define the seven factors proposed by Cloninger and colleagues.
Method
Respondents
The participants included 946 men and women in the National Institute on Aging’s Baltimore Longitudinal Study of Aging
(27). The participants in this study are generally healthy, well-educated, community-dwelling volunteers who visit the Gerontology Research Center every 2 years for biomedical and psychosocial testing. After complete description of the study to the participants, written informed consent was obtained. The subjects who consented to participate consisted of 478 men aged 21 to 94 years (mean=62.1, SD=15.5) and 468 women aged 20 to 93 years (mean=55.4, SD=15.5). The ethnic composition of the total sample was 83.5% white (N=790), 13.1% African American (N=124), and 3.4% other (including Asian Americans, Native Americans, and Hispanics, N=32). The mean education level of the sample was 16.8 years (SD=2.4). DNA was available for only 587 participants (321 men and 266 women). All 587 were genotyped for the D4DR polymorphism. DNA and funds for 5-HTTLPR genotyping were available for 425 participants (217 men and 208 women).
Personality Assessment
The Temperament and Character Inventory is a 240-item, true-false, self-report questionnaire that measures seven dimensions of personality, including the original three Tridimensional Personality Questionnaire scales (or temperaments) of novelty seeking, harm avoidance, and reward dependence
(4,
5). The Temperament and Character Inventory also includes a fourth temperament dimension, persistence, which was originally subsumed under reward dependence, and three character dimensions: self-directedness, cooperativeness, and self-transcendence. The Temperament and Character Inventory also measures 25 more specific traits that define the temperament and character dimensions. Details regarding the development, reliability, and factor structure of the Temperament and Character Inventory are provided elsewhere
(4). Coefficient alpha, an index of internal consistency, reflects the degree to which items in a scale are tapping similar content. For the present sample, the coefficient alphas were 0.76, 0.86, 0.70, 0.60, 0.83, 0.80, and 0.87 for novelty seeking, harm avoidance, reward dependence, persistence, self-directedness, cooperativeness, and self-transcendence, respectively.
D4DR Polymorphism Genotyping
Genomic DNA was extracted from whole blood by standard techniques. The yield of the genomic DNA was assessed by electrophoresis: 2 ml of DNA and a HindIII-digested l standard DNA and genomic DNA concentration standards were subjected to electrophoresis on a 1% agarose gel containing 0.5 mg/ml of ethidium bromide, with subsequent visualization by ultraviolet transillumination. Oligonucleotide primers used in the primary amplification reaction were synthesized by means of standard methods. The positive control included a DNA sample of the most common allele, D4DR 4,4, in parallel with every set of test samples. The amplification reaction contained the following in a final volume of 60 ml: 25–250 ng of DNA, 50 pmol of each primer (as already described), 10% DMSO and buffer, nucleotides (200 mM each of dATP, dTTP, and dCTP and 100 mM each of dGTP and 7-deaza-dGTP), and Taq polymerase and buffer. Initial denaturation of the genomic DNA template was accomplished by heating at 95˚C for 5 minutes. Each of 30 cycles of amplification included annealing for 40 seconds at 54˚C, extension of the primers at 72˚C for 1 minute, and denaturation at 95˚C for 45 seconds. The amplified samples (15–20 ml) were loaded onto a 1.5% agarose gel and electrophoresed at 100 V for 2 to 3 hours. Each set of gel samples included a DNA amplification size marker, the D4DR 4,4 amplification control, a D4 amplification ladder, a DNA negative control, and the test samples. The DNA fragments were visualized on an ultraviolet transilluminator and recorded by photographing the gel.
We used several approaches to defining the short versus long D4DR groupings reported in the literature
(7,
8). The first approach defined the short polymorphism by the absence of the exon III seven-repeat allele and the long polymorphism by the presence of any seven-repeat allele. Because four- and seven-repeat alleles account for the majority of alleles in the population, a second approach compared the 4,4 genotype to the 4,7 genotype. A third approach defined the short polymorphism as two copies of two to five exon III repeats (
s/s genotype) and the long polymorphism as one or two copies of six to eight exon III repeats (
s/l and
l/l genotypes). A fourth approach contrasted the
s/s,
s/l, and
l/l genotypes. We also attempted to replicate the finding of Ekelund et al.
(21) that the two- and five-repeat alleles were associated with novelty seeking.
5-HTTLPR Polymorphism Genotyping
Genomic DNA was extracted from whole blood by standard techniques. Polymerase chain reaction primers and conditions were as described by Lesch et al.
(9). Briefly, primers flanking 5-HTTLPR and corresponding to the nucleotide positions –1416 to –1397 and –910 to –888 of the serotonin transporter gene 5¢-flanking regulatory region were used to generate 484- or 528-base-pair fragments. This polymerase chain reaction amplification was performed in a final volume of 30 ml consisting of 50 ng of genomic DNA, 2.5 mM deoxyribonucleotides, 0.1 mg of sense and antisense primers, 10 mM Tris HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl
2, and 1 U of Taq DNA polymerase. Annealing was carried out at 61˚C for 30 seconds, extension at 72˚C for 1 minute, and denaturation at 95˚C for 30 seconds for 35 cycles. The fragments were separated by agarose gel electrophoresis using 4% gels, and the genotypes were screened by ethidium bromide staining. DNA from 60 randomly selected subjects was sent to an independent laboratory to confirm the veridicality of our 5-HTTLPR assays. Perfect (100%) interlaboratory agreement was obtained.
We used two approaches defining the short and long 5-HTTLPR groupings reported in the literature
(9,
23). In the first approach, participants with either one or two copies of the short variant of 5-HTTLPR were grouped together (
s/s and
s/l genotypes) and were contrasted to participants homozygous for the long variant of 5-HTTLPR (
l/l genotype). The second method contrasted the
s/s,
s/l, and
l/l genotype groups.
Statistical Analysis
All statistical tests were carried out by using SAS software, release 6.12 (SAS, Cary, N.C.). Associations between genotype group and scores on the Temperament and Character Inventory scale were assessed by one-way analysis of variance (ANOVA). Because concerns about ethnic differences have been raised in prior studies
(25), we repeated the analyses while controlling for the demographic variables of race (white versus nonwhite), age, and gender. All analyses were repeated within the subsample of white participants.
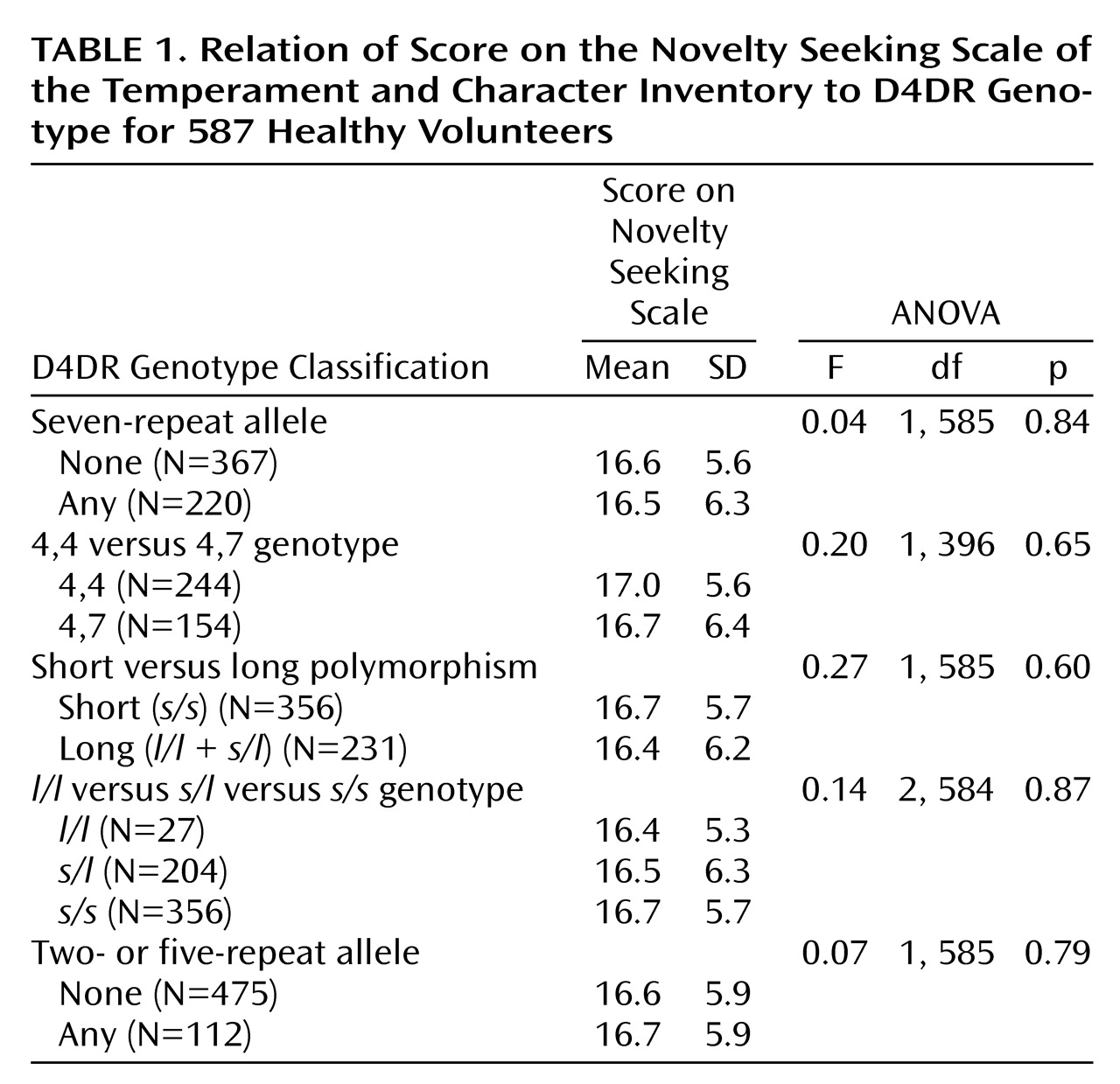
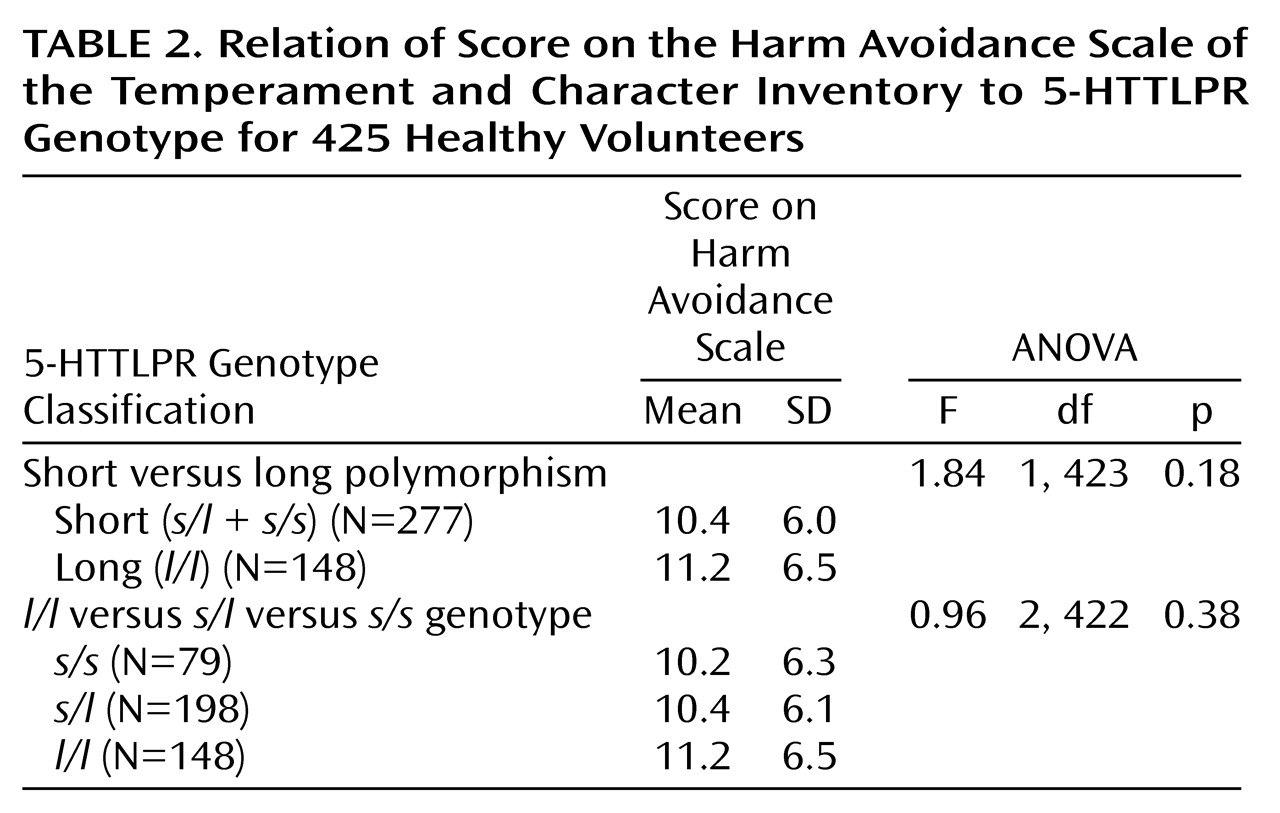
For D4DR, subjects with the absence (short) versus presence (long) of any seven-repeat alleles were compared in terms of scores on the overall Temperament and Character Inventory scale for novelty seeking and on the four subscales within novelty seeking. Additional D4DR genotype comparisons included 1) 4,4 (short) versus 4,7(long); 2) short (s/s) versus long (l/l plus s/l); 3) all three groups (l/l, s/l, and s/s); and 4) presence versus absence of two- and five-repeat alleles. For 5-HTTLPR, the short (s/s plus s/l) and long (l/l) 5-HTTLPR genotype groups were compared on the overall harm avoidance scale and the four harm avoidance subscales. The harm avoidance scores of the three 5-HTTLPR genotype groups (l/l, s/l, and s/s) were also compared.
Following the method Cloninger et al.
(4), we evaluated the phenotypic structure of the Temperament and Character Inventory by conducting a principal components factor analysis of the 25 subscales. Exploratory factor analyses using a variety of indicators (Horn’s parallel analysis, Scree test, and eigenvalue greater than 1.0) suggested that fewer than seven factors should be extracted (Costa et al., manuscript in preparation). However, because the temperament-character model posits seven factors, seven factors were extracted. Varimax and promax rotations were examined.
Results
Analysis of D4DR Genotype
The frequencies of the D4DR polymorphism alleles in the total sample were as follows: allele 2, 8.9%; allele 3, 3.7%; allele 4, 64.3%; allele 5, 1.1%; allele 6, 0.3%; allele 7, 20.4%; and allele 8, 1.1%. One participant had 10 repeats on the second D4DR allele. These distributions are similar to those reported previously
(7,
8). The hypothesized difference in scores on the overall scale for novelty seeking between individuals with no seven-repeat allele (short) and those with any seven-repeat allele (long) was not found (
Table 1). An analysis comparing the scores for novelty seeking of the 4,4 and 4,7 genotype groups was also not significant. Comparison of the groups with long (
l/l plus
s/l) and short (
s/s) polymorphisms also resulted in no significant differences. An analysis of the three D4DR genotype groups (
l/l,
s/l, and
s/s) also resulted in no significant differences in novelty seeking. When participants with the presence of any two- or five-repeat alleles were compared to those without these alleles, no significant effects involving the genotype were obtained. No significant D4DR group differences were found when the analyses were performed on the four subscales of novelty seeking: exploratory excitability, impulsiveness, extravagance, and disorderliness. When the analyses were statistically controlled for the effects of race, age, and gender and when the analyses were repeated within the subsample of white participants, no significant D4DR group differences emerged for novelty seeking or its subscales.
Analysis of 5-HTTLPR Genotype
The frequencies of 5-HTTLPR alleles in the total sample were 58% for the long allele (
l) and 42% for the short allele (
s). The three genotype frequencies were distributed according to Hardy-Weinberg equilibrium (
l/l=0.35,
s/l=0.47, and
s/s=0.18) and are similar to those reported previously
(28,
29). Contrary to findings in a prior report
(9), when the long (
l/l) and short (
s/l plus
s/s) genotype groups were compared on the harm avoidance dimension of the Temperament and Character Inventory, no significant effect for genotype group was obtained (
Table 2). No significant 5-HTTLPR genotype group differences were found for the four subscales for harm avoidance: anticipatory worry (F=0.47, df=1, 423, p=0.49), fear of uncertainty (F=1.96, df=1, 423, p=0.16), shyness with strangers (F=1.06, df=1, 423, p=0.30), and fatigability (F=1.09, df=1, 423, p=0.30). An analysis of the three 5-HTTLPR genotype groups (
l/l,
s/l, and
s/s) also indicated no significant differences in scores for harm avoidance. Analyses that statistically controlled for the effects of race, age, and gender and analyses within the subsample of white participants also resulted in no significant genotype differences for harm avoidance or its subscales.
Principal Components Analysis
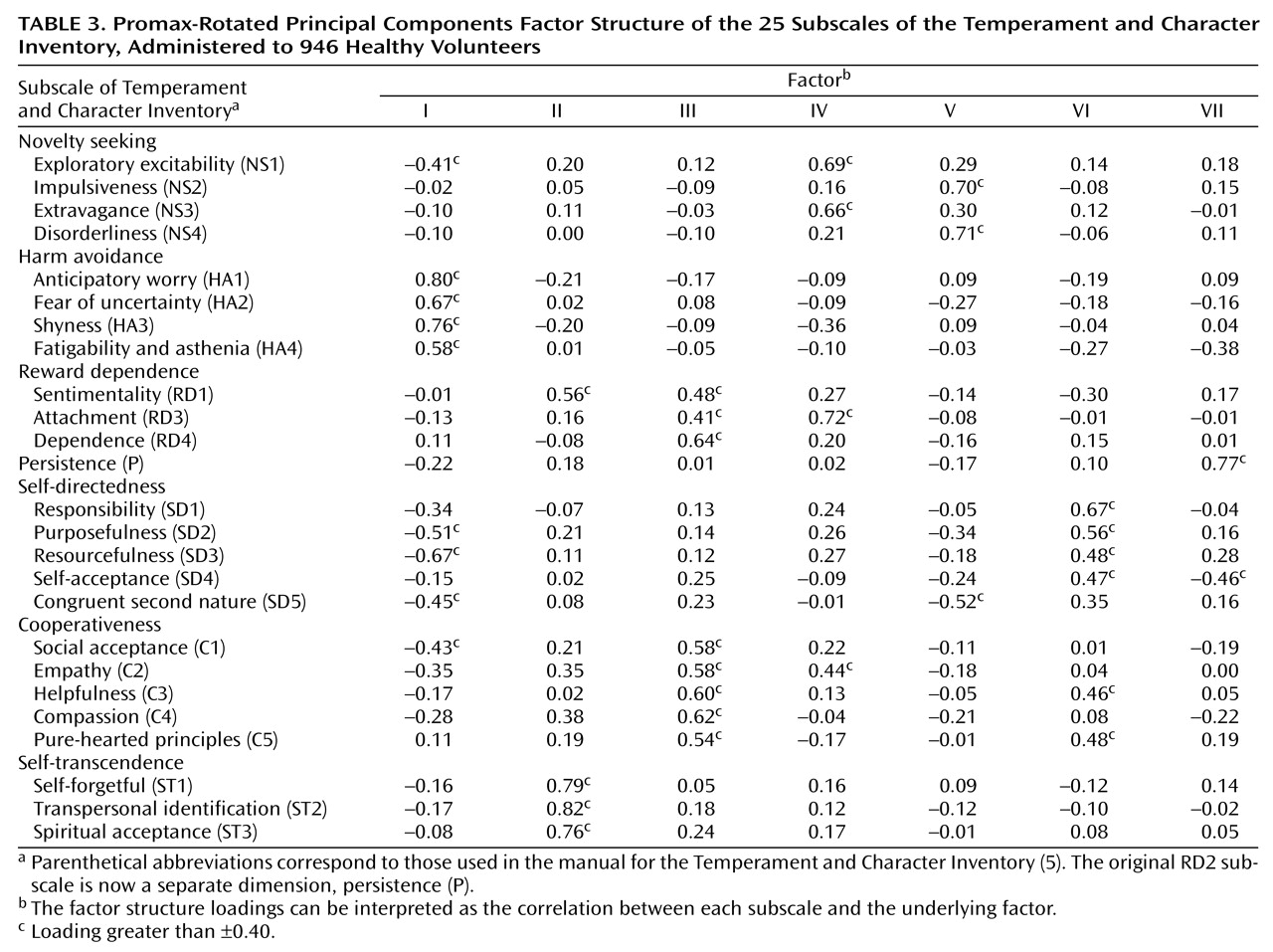
The results of the varimax and promax rotations were similar. For comparison to the table in the Temperament and Character Inventory Manual
(5),
Table 3 presents the factor structure loadings from the promax-rotated principal components analysis of scores on the 25 subscales of the Temperament and Character Inventory for the participants in our study. The factor structure loadings can be interpreted as the correlation between each subscale and the underlying factor. The observed factor structure of the Temperament and Character Inventory instrument does not correspond to the hypothesized seven-factor structure of the temperament-character model. In particular,
Table 3 reveals two recurring problems with the Temperament and Character Inventory. First, according to the temperament-character model, all the subscales of each temperament or character factor should load on the same factor. Yet, several subscales have split or joint loadings. For example, the subscales for novelty seeking define two separate factors (IV and V), and the subscales for reward dependence have their chief loadings on three different factors (II, III, and IV). Second, several of the factors are defined by an admixture of subscales from the temperament and character domains. For example, factor I is clearly defined by the four harm avoidance subscales but also has significant inverse loadings from three of the five self-directedness subscales. Factors II and III combine the temperament scales of reward dependence with the character scales of self-transcendence and cooperativeness, respectively. The conceptual distinction between temperament and character is not empirically supported in these analyses of the Temperament and Character Inventory.
Discussion
In this investigation we found no support for the temperament-character model on either the biological or psychological level. Recall that the dimensions of the psychobiological model of temperament are hypothesized to be regulated neurochemically by a complex network of brain connections, and the model was used as a guide in the development of the Temperament and Character Inventory. The model hypothesizes that genetic variability in dopamine transmission plays a crucial role in the neuromodulation of behavioral activation in response to novelty (novelty seeking traits), and genetic variability in serotonin transmission is the major neuromodulator of the brain’s inhibition system (harm avoidance traits)
(5). At the genetic level, novelty seeking was not related to any of the D4DR alleles (e.g., long versus short, as originally proposed, or two and five repeats, as recently proposed by Ekelund et al.). Similarly, the serotonin transporter gene was not related to variations in the temperament dimension of harm avoidance.
Previous discussions
(30,
31) of the failure to replicate the phenotypic and genotypic links focused only on the selection of candidate genes. The present findings suggest that it is also possible that the temperament-character model is incorrect. That interpretation is suggested by the failure of the principal components analysis to replicate the hypothesized factors. If the subscales of the Temperament and Character Inventory share some genetic basis, they should covary to define a common factor. The four subscales for harm avoidance do cohere, which is consistent with the notion that they share a genetic basis. However, it is unlikely that the subscales for novelty seeking or reward dependence have a common genetic basis, because they do not define a single factor. These results are consistent with those in a recent study by Ball et al.
(32), who also failed to uncover the hypothesized factor structure of the Temperament and Character Inventory. The dimensions of the Temperament and Character Inventory do not represent a “simple genetic architecture,” as Cloninger and colleagues
(4) claimed.