Prediction parameters were also compared between the groups with schizophrenic and affectively ill parents to assess unique risk for offspring of parents with schizophrenia and between schizophrenia-related psychoses and other axis I outcomes to assess uniqueness to schizophrenia-related psychoses.
Method
Subject Selection and Diagnostic Procedures
The New York High-Risk Project recruited offspring of schizophrenic, affectively ill, and psychiatrically normal parents in 1971–1972 and in 1977–1979. Mentally ill parents were identified from admissions to state psychiatric hospitals in lower New York State, interviewed with the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L)
(23), and diagnosed according to Research Diagnostic Criteria (RDC)
(24). Normal comparison parents were free of major mental disorders and prior psychiatric treatment history
(20).
The offspring were white, English-speaking, a mean age of 9.29 years (SD=1.76), and free of mental retardation, major psychiatric disorders, or treatment for emotional problems at recruitment. Subjects received six rounds of evaluations approximately 3 years apart.
Written informed consent was obtained from the parents for themselves and their minor children at the first contact with the family and from each individual participant at all subsequent examinations.
SADS-L interviews were administered during the fourth through sixth evaluations, at which the subjects were a mean age of 19.70 years (SD=1.83), 23.20 (SD=2.12), and 27.30 (SD=2.16), respectively. Lifetime axis I diagnoses and estimates of onset age were based on the interviews and clinical records. For subjects with new onsets since the sixth evaluation, assessments were updated to autumn 1995, at which point the subjects were a mean age of 30.70 years (SD=3.26). Details concerning interview procedures and reliability have been previously described
(20,
21).
For this report, adulthood axis I disorders were hierarchically classified into three categories: 1) schizophrenia-related psychoses (schizophrenia, unspecified psychosis, and “mainly schizophrenic” schizoaffective disorder [per the RDC distinction]), 2) major affective disorders (“mainly affective” schizoaffective disorder [per the RDC distinction], bipolar I and bipolar II disorder, and major depression); and 3) other major axis I disorders (hypomania, intermittent depression, anxiety disorders, and substance abuse disorders). Subjects were counted only once, regardless of comorbidity, in the hierarchal order.
Only those offspring with complete childhood neurobehavioral data and diagnostic assessments in adulthood were included in this report. Of the original 358, 34 had died or could not be traced to adulthood. Of the 324 followed subjects, 55 had incomplete neurobehavioral data because of equipment failure or administration of different tests. Thus, this report concerns 269 subjects. Adulthood axis I diagnoses among the 79 offspring of schizophrenic parents included schizophrenia-related psychoses (15.2%, N=12), major affective disorders (35.4%, N=28), and other major axis I disorders (16.5%, N=13); 32.9% (N=26) had no disorder. Among the 57 offspring of affectively ill parents, 7.0% (N=4) had schizophrenia-related psychoses, 45.6% (N=26) had major affective disorders, and 21.1% (N=12) had other major axis I disorders in adulthood; 26.3% (N=15) had no disorder. Finally, schizophrenia-related psychoses, major affective disorders, and other major axis I disorders affected 0.8% (N=1), 30.1% (N=40), and 27.8% (N=37) of the 133 offspring of normal parents, respectively; 41.4% (N=55) had no disorder.
Childhood Neurobehavioral Performance
The cognitive batteries have been described in detail
(22). Both groups (i.e., those recruited in 1971–1972 and in 1977–1979) were given variants of the Continuous Performance Test, a test of visual sustained attention
(25) widely used in schizophrenia research.
Other cognitive tasks employed were the Attention Span Task, a measure of short-term memory storage capacity based on procedures reported in studies of schizophrenic patients
(26) and the digits forward and backward components of the Digit Span subtest from the Wechsler Intelligence Scale for Children (WISC or WISC-R). (For offspring recruited in 1977–1979, the Visual Aural Digit Span Test
[27] was substituted for the Attention Span Task and Digit Span tasks to test immediate auditory and visual recall.)
Neuromotor assessment was based on a 31-item version of the Lincoln-Oseretsky Motor Development Scale
(28).
Two types of summary measures were developed: the Attention Deviance Index, a measure of global attention as described previously (17, 22), and composite variables selected by using hypotheses that were based on the structure of the tests and entered into factor analyses with varimax rotation
(29). The factor analyses were carried out separately for the two recruitment groups, and the factor structures were identical. The resulting five factors were errors and correct responses (both from Continuous Performance Test variables), memory (from the Digit Span, Attention Span Task, and Visual Aural Digit Span Test), and fine motor and gross motor skills (from the Lincoln-Oseretsky Motor Development Scale).
Statistical Analyses
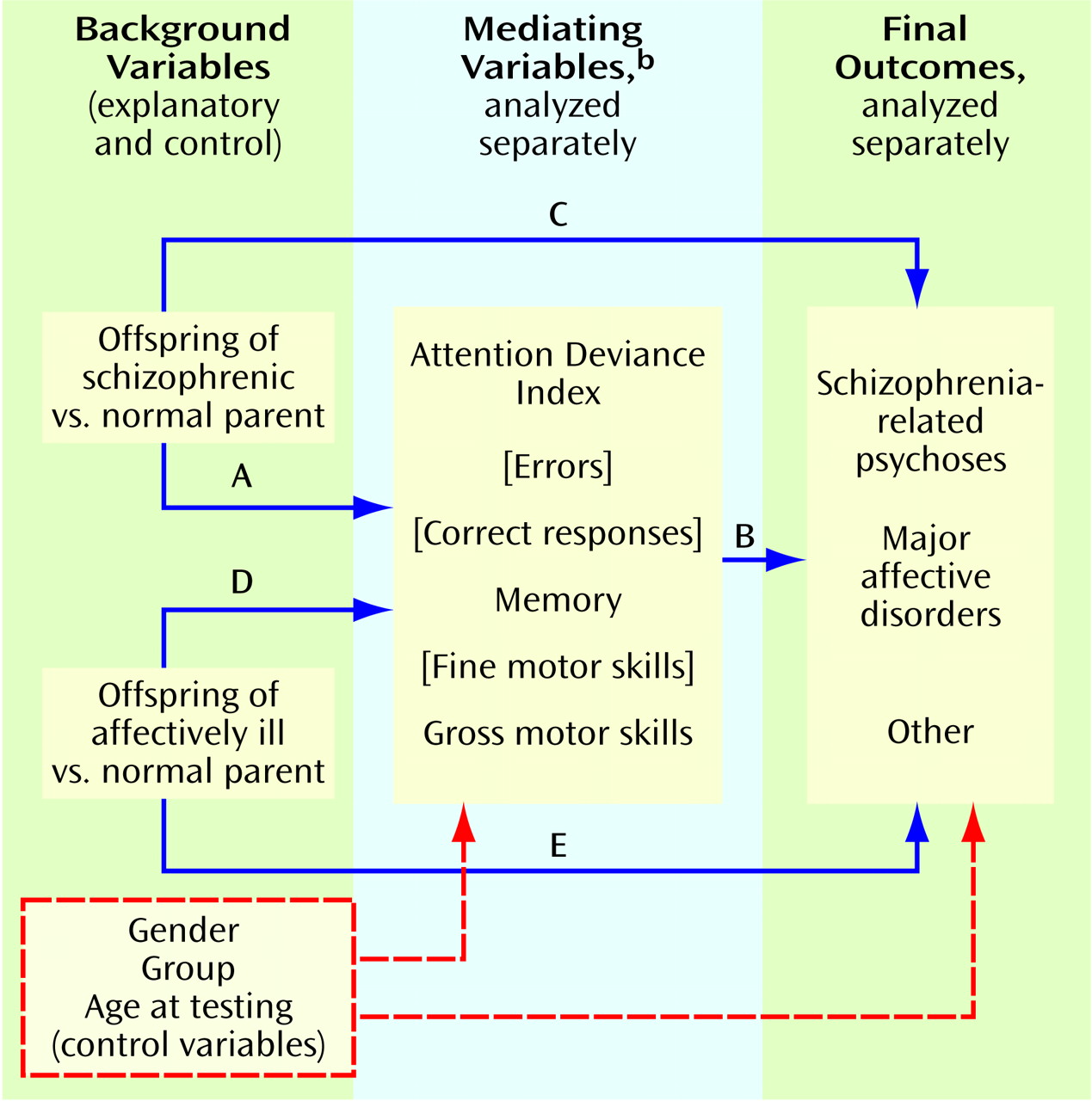
We developed theoretical models to be tested separately for each of six mediating variables (i.e., performance on the Attention Deviance Index and the aforementioned five factors). The model for each mediating variable generated two equations for which explanatory variables (parental psychiatric status) and control variables (gender, recruitment sample [1971–1972 versus 1977–1979], and age at testing) were included as background variables. The first equation, a linear regression, related parental psychiatric status and control variables to childhood neurobehavioral performance on the mediating variable. The second equation, a logistic regression, related parental psychiatric status, control variables, and performance on the mediating variable to the psychiatric outcome. Although schizophrenia-related psychoses was the main psychiatric outcome of interest, separate analyses were also run for major affective disorders and for other major axis I disorders.
In the theoretical model (
Figure 1), two paths of main interest were 1) the effect of being an offspring of a schizophrenic parent versus being an offspring of a psychiatrically normal parent in relation to childhood neurobehavioral performance on the mediating variable (path A) and 2) the relationship of performance on the mediating variable to psychiatric outcome (path B). Paths A and B were assessed in the linear and logistic regressions, respectively. Another critical pathway assessed in the logistic regression analysis was the direct effect of having a schizophrenic parent versus a normal parent in relation to the outcome (path C). Paths D and E (analogous to paths A and C, respectively, but with the effect of being an offspring of an affectively ill parent replacing that of being an offspring of a schizophrenic parent) were also examined through linear and logistic regression equations.
The strongest genetic model would be one showing that 1) being an offspring of a schizophrenic parent significantly affects performance on the mediating variable (path A)—thereby suggesting that this variable could be a phenotypic indicator of the genetic liability to schizophrenia—and 2) performance on the mediating variable and offspring-of-schizophrenic-parents status significantly predict future development of schizophrenia-related psychoses (paths B and C, respectively). Such a model would be consistent with the fact that some, but not all, of the phenotypic indicators have been identified and, therefore, that familial background (path C) remains as a significant direct effect on the development of schizophrenia-related psychoses. Significant findings for paths A and B only present a weaker case for the genetic model, since the schizophrenic-parent effect has no explanatory power for the development of schizophrenia-related psychoses. A model with significant paths A and C only would suggest that, although the mediating variable might be a liability indicator, it is not useful as a predictor of a schizophrenia-related psychosis outcome. A model with significant paths B and C only might suggest that, whereas both performance on the mediating variable and the schizophrenic-parent effect predict the outcome, the mediating variable is not an indicator of genetic liability. This model might be found for environmental factors that interact with a genetic liability in the development of schizophrenia.
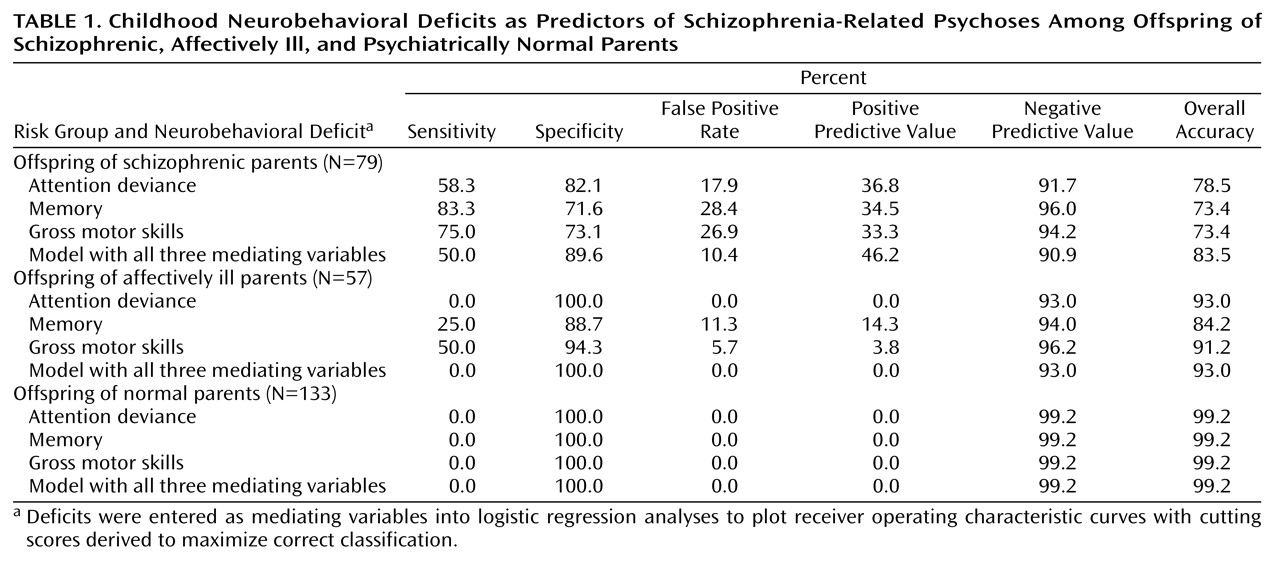
Our goal was to compare the sensitivity and specificity of models in which regression coefficients for both paths A and B were significant as predictors of the onset of schizophrenia-related psychoses (i.e., the models in which the mediating variable represents a potential endophenotype as well as predictor of later illness.) On the basis of predictive probabilities yielded by the logistic regression analyses, receiver operating characteristic curves were plotted. From these curves we derived cutting scores to maximize the correct classification rates for schizophrenia-related psychoses, i.e., scores that maximized sensitivity and specificity of the model in predicting schizophrenia-related psychoses for offspring of schizophrenic parents. Sensitivity represents the percentage of subjects with schizophrenia-related psychoses correctly classified by the cutting score, and specificity represents the percentage of subjects without schizophrenia-related psychoses who were correctly classified as not developing schizophrenia-related psychoses. Note that since 1–specificity equals the false positive rate (i.e., the proportion of subjects falsely predicted to develop schizophrenia-related psychoses), maximizing specificity ensures that the false positive rate is minimized.
Results
Mean performance on each of the six mediating variables was significantly worse in the offspring of schizophrenic parents than in the other groups. However, the models for errors, correct responses, and fine motor skills were not significantly related to onset of schizophrenia-related psychoses. These variables were therefore dropped. (No mediating variable was significantly related to onset of major affective disorders or other major axis I disorders.)
The contrast between offspring of schizophrenic parents and offspring of normal parents (path A) was significant in the models involving the three remaining neurobehavioral variables (Attention Deviance Index: t=4.87, df=273, p<0.001; memory: t=–2.02, df=264, p=0.05; gross motor skills: t=–4.22, df=310, p<0.001). For all three models, a significant direct relationship with schizophrenia-related psychoses was seen for both performance on the mediating variable (path B) and the contrast between offspring of schizophrenic parents and offspring of normal parents (path C): (Attention Deviance Index model, path B: Wald χ2=3.70, df=1, p=0.054; path C: Wald χ2=7.83, df=1, p=0.005; odds ratio=20:1. Memory model, path B: Wald χ2=3.66, df=1, p=0.056; path C: Wald χ2=9.05, df=1, p=0.003, odds ratio=25:1. Gross motor skills model, path B: Wald χ2=7.48, df=1, p=0.006; path C: Wald χ2=7.97, df=1, p=0.005, odds ratio=20:1). The combination of significant regression coefficients for paths A, B, and C is consistent with the genetic model and suggests that the mediating neurobehavioral variables may be phenotypic indicators of the genetic liability to schizophrenia-related psychoses and that the variables together with offspring-of-schizophrenic-parents status predict the future development of these disorders.
The contrast between offspring of affectively ill parents and offspring of normal parents (path D) was significant only for gross motor skills (t=–1.98, df=310, p=0.05), but path E—the direct effect of an affectively ill parent on development of schizophrenia-related psychoses—was not significant, which indicated a weak genetic model.
Table 1 presents sensitivity, specificity, and false positive rates for prediction of the development of schizophrenia-related psychoses, derived from the aforementioned receiver operating characteristic curves. Positive and negative predictive values (i.e., the percentages of subjects with impairment on the mediating variable who developed schizophrenia-related psychoses and of subjects without impairment who did not develop such disorders, respectively) and overall accuracy rates (true positive plus true negative subjects) are also presented. For offspring of schizophrenic parents, sensitivity in identifying future development of schizophrenia-related psychoses was highest for the regression model with memory (83.3%) as the mediating variable and lowest for the model with Attention Deviance Index performance (58.3%). Conversely, false positive rates in the group of offspring of schizophrenic parents were lowest for the Attention Deviance Index model and not as good for the models with memory and gross motor skills as mediating variables. Positive predictive values and overall accuracy rates were nearly the same for offspring of schizophrenic parents with all three mediating variables.
Low sensitivity and false positive rates for prediction of schizophrenia-related psychoses in the offspring of affectively ill and psychiatrically normal parents suggest that deficits in each mediating variable representing neurobehavioral performance are comparatively unique to risk for schizophrenia. For example, the total prevalence of impairment in the offspring of schizophrenic parents compared with offspring of affectively ill parents was 24% versus 0%, respectively, for attention, 37% versus 12% for memory, and 34% versus 9% for motor skills.
We also examined agreement across the three prediction models. In the group of offspring of schizophrenic parents, 91.7% (N=11 of 12) of those with schizophrenia-related psychoses were correctly classified by at least one model. As
Table 1 shows, all three models together identified half of the subjects with schizophrenia-related psychoses (50% sensitivity, 46% positive predictive value) but also tagged 10% of the subjects without schizophrenia-related psychoses as false positive. Among the offspring of affectively ill parents, no subjects were predicted to develop schizophrenia-related psychoses by all three models, and only one was predicted by two models.
Discussion
Deficits in attention, memory, and neuromotor functions were noted in early formal descriptions of schizophrenia
(30) and subsequently documented in experimental studies of patients and their relatives
(10,
11,
15). This report suggests that such dysfunctions clearly meet at least three of the criteria that have been established to qualify a variable as a phenotypic indicator
(2–
4). In this study, the deficits were 1) present before clinical symptoms and thus independent of the illness state; 2) substantially more prevalent in relatives of schizophrenic patients than in relatives of comparison subjects; and 3) comparatively specific to risk for schizophrenia versus risk for affective disorders, in that they exhibited greater prevalence among offspring of schizophrenic parents than among offspring of affectively ill parents. The neurobehavioral variables were also specifically related to schizophrenia-related psychoses as the psychiatric outcome rather than to other outcomes.
A fourth criterion—longitudinal stability and persistence of impairment—was seen for two of the variables as verbal memory was assessed and age-appropriate versions of the Attention Deviance Index
(31) were administered over several evaluations. Gross motor skill was tested only in childhood, but other studies
(32) suggest that neuromotor deficits continue to older ages.
The chief focus here, however, was on the effectiveness of the three measures. Each showed comparatively good accuracy of classification, with rates at the upper end of the range previously reported in high-risk research, but the sensitivity-specificity balance differed among the variables. Sensitivity, in correctly predicting schizophrenia-related psychoses, was unusually high for verbal memory (83%) and gross motor skills (75%). Comparatively, the Attention Deviance Index had a weaker sensitivity rate but fewer false positive classifications among offspring of schizophrenic parents who did not develop schizophrenia-related psychoses. Thus, implications for use in genetic or intervention research differ for these variables.
Because gene search studies are especially vulnerable to false positive classification of relatives as susceptibility gene carriers
(6), the Attention Deviance Index may be the most useful of the three variables for such studies. Indeed, a “diagnostic accuracy” analysis
(6) that compared a number of personality disorders and neurobehavioral variables (not including memory or gross motor skills) that had been proposed as indicators of the schizophrenia genotype suggested that the Attention Deviance Index might prove the most useful for increasing informativeness of linkage data. Furthermore, in the present analyses, impairment rates were greater for memory and gross motor skills than for the Attention Deviance Index in offspring of affectively ill parents, thus suggesting better discrimination by the Attention Deviance Index between schizophrenia pedigree members who are at risk for affective disorders and those who are carriers of the schizophrenia genotype. This is important because, given the high population base rate for affective disorders, individuals at risk for these disorders are often included by chance in schizophrenia pedigrees.
Intervention, the long-term but often controversial goal of high-risk research, poses other questions with respect to the level of tolerance for predictive misclassifications. Strategies, such as cognitive intervention therapies, that have little danger of deleterious effects can obviously tolerate higher false positive and lower sensitivity rates (or positive predictive values) than strategies, such as pharmacological interventions, that are potentially more powerful but could produce harmful effects, especially in children or adolescents. On the whole, predictive performance of our three measures, or of most measures reported in high-risk studies, does not seem to support use of the latter types of intervention in young high-risk subjects.
A limitation of the present analyses is that they cannot be directly extrapolated to detection of preschizophrenic individuals in the general population. Since only about 15% of future schizophrenic patients have a schizophrenic first-degree relative, identification of the majority of preschizophrenic individuals for any type of intervention program would need to be based on general population screening. Given the low population base rate of schizophrenia-related psychoses, however, normal control groups in this and other high-risk studies are too small to test an indicator’s capacity as an effective preschizophrenia screen and predictor of schizophrenia-related psychoses in the general population. Thus, this study provides no information about choosing individuals from the general population, even for “benign” interventions.
The most conservative approach to detection either of probable genotypes in schizophrenia pedigrees or of individuals considered for intervention in high-risk research would be one requiring evidence of impairment on multiple “endophenotypic”
(1) measures. In the present analyses, the combination of impairment on all three variables together achieved better classification among the offspring of schizophrenic parents with respect to false positive rate (10%), positive predictive value (46%), and overall accuracy (83%) than any of the variables individually.
The nonpsychotic offspring of schizophrenic parents who were among the 10% falsely classified when all three variables were combined are of interest because they appear to be carriers of some of the susceptibility genes for schizophrenia and may yield information, by default, about other factors that are needed for development of the overt illness. It is possible, of course, that some of these subjects will express the clinical illness in the future. However, they were not younger than the mean age at onset of the subjects with schizophrenia-related psychoses and had not met criteria for probable cluster A disorders, which most subjects with schizophrenia-related psychoses exhibited before onset of psychoses.
A likely possibility—expected from the multifactorial nature of schizophrenia
(1)—is that deficits in attention, memory, and gross motor skills reflect only some of the phenotypic indicators of a large complex of susceptibility genes, as indicated by the significant effect of path C from family background to schizophrenia-related psychoses when the influence of the mediating variables is controlled. Thus, subjects with schizophrenia-related psychoses may exhibit additional as-yet-unidentified phenotypic impairments than the subjects who were false positive. Although several neurobehavioral variables examined during the childhood testing did not relate to schizophrenia-related psychoses, other measures, not yet available at that time (e.g., functional brain imaging, more complete and refined neuropsychological test batteries, etc.) might have been better predictors. Alternatively, false positive subjects may have fewer negative “polygenic potentiators”
(33) or may have experienced less exposure to environmental factors that interact with susceptibility genes for full clinical expression of the illness. Ongoing analyses in the New York High-Risk Project are exploring these possibilities by comparing a number of phenotypic and historical variables among offspring of schizophrenic parents 1) with schizophrenia-related psychoses, 2) without schizophrenia-related psychoses and correctly classified as such (true negative), and 3) without schizophrenia-related psychoses but falsely classified as such.
Childhood manifestation of neurobehavioral deficits by preschizophrenic subjects is consistent with current neurodevelopmental hypotheses
(34,
35), which suggest that early brain damage—resulting from environmental insults, faulty genetic programming, or their interaction—may remain unexpressed as clinical symptoms for many years but may appear as earlier neurobehavioral or other deficits. Cognitive deficits, notably including memory and attentional impairments, with possible roots in early developmental damage are considered hallmark characteristics of schizophrenia. Nevertheless, as demonstrated here, motoric dysfunctions must also be considered in explanations of the pathophysiology of the illness.
It has been proposed that dysfunctional frontal-cerebellar-thalamic circuitry, which appears to underlie memory impairment in schizophrenic patients, could also account for a broad range of other cognitive and motor disturbances
(36,
37). The hypothesized disruption in a central timing process associated with this circuitry is of interest in connection with the present cognitive and motor findings, especially when taken together with the fact that offspring of schizophrenic parents in this study displayed clear temporal deficits on a perceptual duration task (unpublished 1999 manuscript of T. Penney et al.).
On the whole, the present analyses indicate the importance of prospective follow-up of offspring of schizophrenic and other mentally ill parents for identifying early neurobehavioral dysfunctions that are probably endophenotypic indicators of schizophrenia susceptibility genes, strong predictors of schizophrenia-related psychoses in the offspring, and comparatively specific to the liability for schizophrenia contrasted with other psychiatric disorders.