Schizophrenia has increasingly been conceptualized as a neurodevelopmental disorder in which brain organization is somehow altered during development or disrupted before the onset of overt symptoms
(1,
2). The exact timing of the disruption in brain maturation is not known, but researchers speculate that a static lesion occurring in the perinatal period could negatively interact with developmental changes that occur much later, closer to young adulthood, when onset of the disorder typically occurs
(2). Childhood-onset schizophrenia is a much rarer form of the disorder in which the onset of psychotic symptoms occurs before 12 years of age
(3) and before the completion of brain maturation observed in normative studies
(4–
10). Researchers have focused on this schizophrenia subpopulation because patients with childhood-onset schizophrenia are thought to have a more severe form of the same disorder suffered by patients with adult-onset schizophrenia
(11). Researchers have also noted that patients with childhood-onset schizophrenia may be more homogeneous than their adult-onset counterparts, less affected by environmental factors that could have a negative impact on the central nervous system (CNS) (e.g., long-term neuroleptic exposure, chronic illness, substance abuse
[12]).
Extensive research into structural and functional brain abnormalities that could result in psychotic symptoms and neurobehavioral impairments has been conducted with adults who have schizophrenia
(13). The most consistent findings have been abnormally high ventricular and sulcal volumes
(14), hypoplasia of mesial temporal structures
(15), large basal ganglia structures
(16), and low cranial volume
(16). Abnormally low volume of total cortical gray matter has also been reported, with high volumes of white matter
(14). Postmortem studies have revealed cytoarchitectonic abnormalities in brain regions such as the cingulate and dorsolateral prefrontal cortex
(17). Taken together, these findings support the hypothesis that certain abnormalities in brain morphology occur before the onset of psychotic symptoms and are, perhaps, developmental in nature.
Although few in number, studies of brain morphology in childhood-onset schizophrenia have revealed findings similar to those in the studies of adults. Specifically, low brain volume and high basal ganglia volume
(18,
19), high ventricular volume
(20,
21), and callosal abnormalities
(22) have been observed. Temporal lobe abnormalities have also been reported, indicating that children with childhood-onset schizophrenia have abnormally large superior temporal gyri (relative to brain size) and lack the normal right-greater-than-left hippocampal asymmetry
(23). Longitudinal assessment of subjects with childhood-onset schizophrenia and serially assessed comparison subjects have revealed that the patients with childhood-onset schizophrenia have progressively larger ventricles
(21), progressively smaller temporal lobe structures
(24), and progressive cortical differences in the temporal, frontal, and parietal regions
(25). Notably, convincing evidence of volumetric change (e.g., ventricular enlargement) has not been consistently supported by longitudinal imaging studies of adults with schizophrenia
(26).
While the volumetric magnetic resonance imaging (MRI) studies just discussed have provided valuable insights into the pathogenesis of childhood-onset schizophrenia, researchers have, thus far, been hindered by the relative labor-intensiveness of volumetric image analysis techniques. The image analysis methods that are traditionally used to assess functional imaging data may be useful, and perhaps more efficient, in localizing abnormalities in the brain structure of children with schizophrenia. With statistical parametric mapping (SPM) techniques, the whole brain can be analyzed at once without tedious slice-by-slice region definition, and brain regions without clear gyral or structural boundaries (e.g., white matter) can also be assessed. A similar approach was first reported by Andreasen et al.
(27), who examined structural abnormalities in adult-onset schizophrenia on a voxel-by-voxel basis. More recently, SPM has been used to localize the relatively subtle structural maturational changes known to be occurring between childhood and adolescence
(9,
28) and between adolescence and young adulthood
(10), to assess neurobehavioral correlates of brain abnormality in adults with schizophrenia
(29), and to examine structural abnormalities in unipolar depression
(30) and schizophrenia
(31).
The goal of this study was to explore brain abnormalities in a group of children and adolescents with early-onset schizophrenia spectrum disorder by using SPM (specifically, SPM96 software
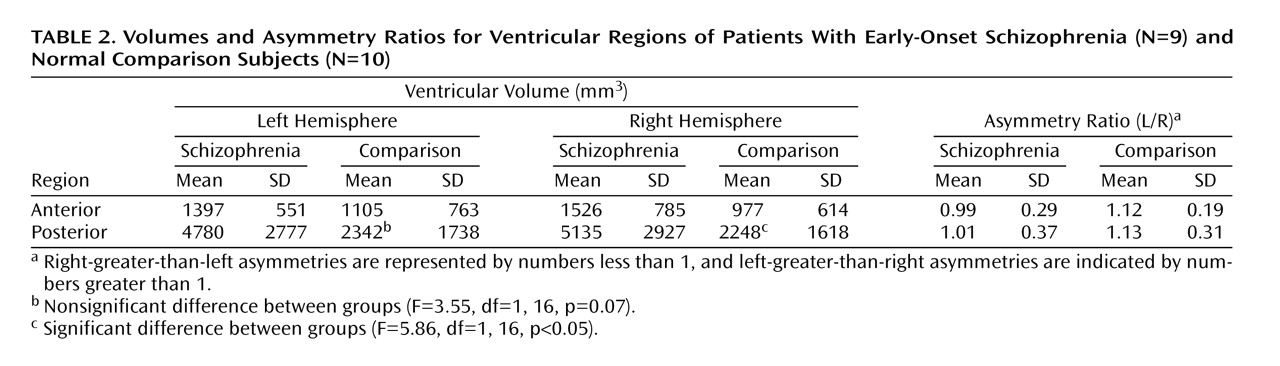
[32]) in conjunction with volumetric techniques. Because high ventricular volume is the most robust finding in the literature on adults with schizophrenia
(13) and has also been observed in childhood-onset schizophrenia, we predicted that we would see evidence for large ventricles if we used these novel methods and that the abnormalities would be better localized than in prior volumetric studies.
Method
Subjects
Nine subjects with early-onset schizophrenia and 10 comparison subjects were examined. Age, gender, clinical information, and handedness are shown in
Table 1. No significant group differences were found for age, gender, or handedness, although there was a 2-year mean age difference between groups (the subjects with schizophrenia were older). The children and adolescents with schizophrenia were recruited from ongoing studies in the Childhood-Onset Schizophrenia Program at the University of California, Los Angeles (UCLA), and for most (N=7) the onset of the disorder had occurred before age 13. One subject’s medical records indicated that the age at diagnosis was 16 years, which is still much younger than the average onset age for this disorder. The sources of subjects for these studies included inpatient and outpatient facilities at the University of California, Los Angeles, and residential treatment facilities throughout the Los Angeles area. Normal children and adolescents were recruited as comparison subjects for ongoing neurodevelopmental studies at UCLA or were friends of the investigators. All of the normal children were screened for neurological impairments and for any history of learning disability or developmental delay.
Psychiatric diagnoses were based on structured interviews (Schedule for Affective Disorders and Schizophrenia for School-Age Children
[33]) of the children and their parents. The interviews were conducted by psychiatrists or doctoral-level clinical psychologists. Diagnoses of schizophrenia, schizoaffective disorder—depressed, or schizophreniform disorder were made on the basis of DSM-III-R criteria. Children were excluded from the study if they had full-scale IQs of less than 70, had any history of CNS disease that could produce psychotic-like symptoms, or had taken drugs that could produce psychotic-like symptoms. Written informed consent was obtained from all subjects and their parents.
Image Acquisition and Processing
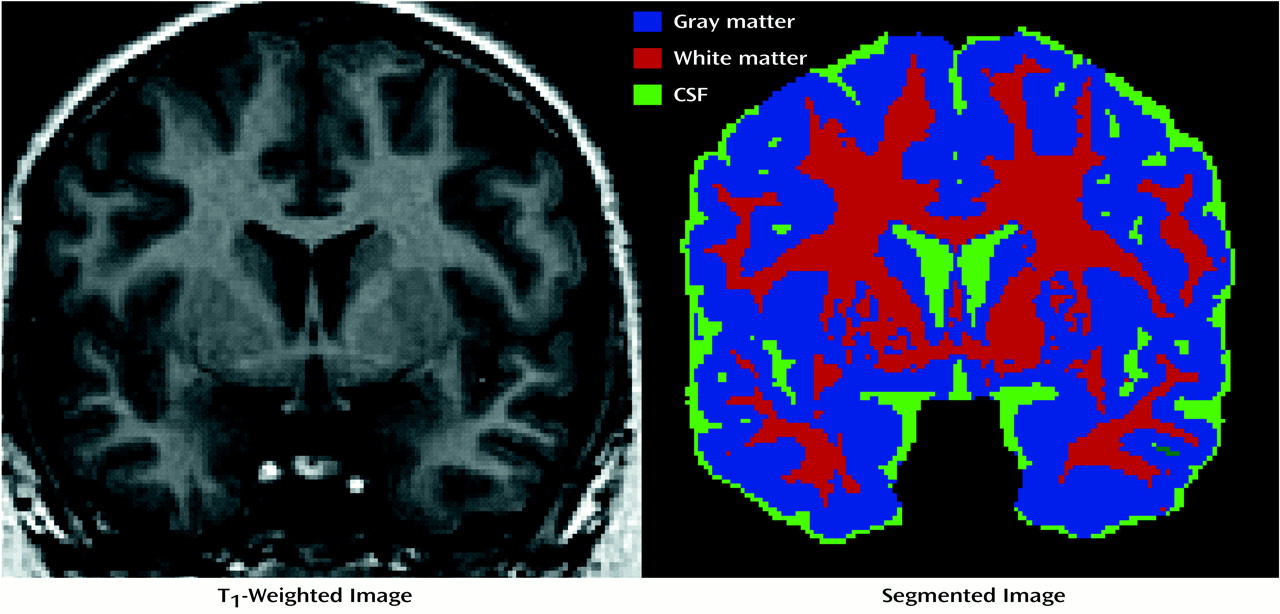
All subjects completed MRI performed on a 1.5-T Signa magnetic resonance scanner (GE Medical Systems, Milwaukee) that used a coronal T
1-weighted spoiled gradient/recall acquisition in the steady state (spoiled GRASS) sequence of 42/5/1, 43/6/1, or 43/7/1 (TR/TE/excitations). All image data sets had a slice thickness of 1.4 mm without gaps, a flip angle of 35°, and a matrix size of 256 ≥ 192 mm (sample shown in
Figure 1).
We have described the methods for image processing in detail elsewhere
(9) and will only briefly summarize them here. The image data sets were subjected to the following preprocessing analyses: rigid-body reslicing of the volume into a standard orientation, interactive isolation of supratentorial cranial regions from surrounding extracranial and infratentorial tissue, three-dimensional digital filtering to reduce inhomogeneity artifact
(34), tissue segmentation based on semiautomated algorithms (V. Kollokian, 1996 master’s thesis), and finally, scaling into the International Consortium for Brain Mapping’s 305 standard space
(35,
36) by means of an automated 12-parameter affine (linear) transformation
(37).
The image data were resliced into a standard orientation by trained operators “tagging” 10 standardized anatomical landmarks in each subject’s image data set that corresponded to the same 10 anatomical landmarks defined on the International Consortium for Brain Mapping average 305 brain
(35). This reslicing step was performed only to provide image analysts with a standard view of the brain, making it easier to apply standardized rules for defining cerebral and noncerebral regions.
Semiautomated tissue segmentation was then conducted for each volume data set to classify voxels, on the basis of signal value, as most representative of gray matter, white matter, or CSF. A simple minimum-distance classifier was used
(35) because it provided the best results (for this T
1-weighted imaging protocol) in a qualitative comparison of different algorithms for tissue segmentation. We have presented a detailed discussion of the reliability and validity of the protocol for tissue segmentation elsewhere
(9). A sample image of a segmented tissue slice is contained in
Figure 1.
The tissue-classified images were then used to aid in creating a mask of the brain that excluded the cerebellum and nonbrain tissue.
The brain masks were used to apply a three-dimensional spatial filter to remove low-frequency changes, or “drifts,” in signal value across the volume. The images were resubmitted to the minimum-distance classifier, resulting in skull-stripped, radio-frequency-corrected, tissue-segmented volumes for each subject.
The tissue-classified supratentorial volume from each subject was scaled into a standard space by using an automated 12-parameter affine (linear) transformation
(37). This procedure, in effect, removes variability due to differences in head size.
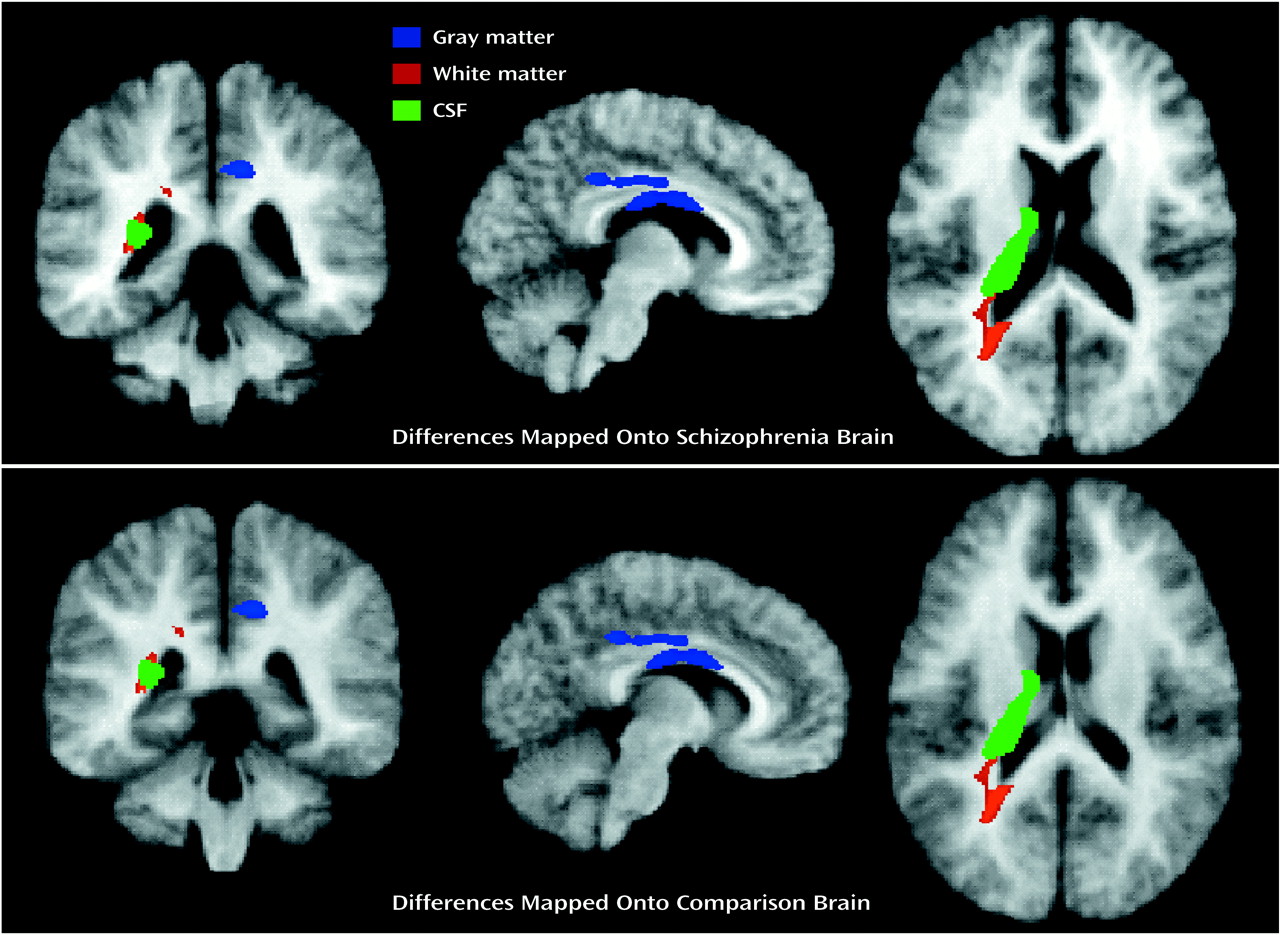
After spatial normalization, the segmented volumes were “binarized” to include 1) only voxels that were classified as gray matter (blue regions in
Figure 1), constituting one data set, 2) voxels that were classified as white matter (red regions in
Figure 1), another data set, and 3) voxels that were classified as CSF (green regions in
Figure 1), the final data set, for importation into the SPM96 program
(32). The resultant image files were binary, with values of 0 or 1 indicating the presence or absence of tissue at each voxel. The spatially normalized gray and white matter and CSF maps were imported into SPM96 and smoothed with an 8-mm full width at half maximum isotropic Gaussian kernel to create the spectrum of gray/white matter or CSF intensities needed for the statistical analyses.
Statistical Analysis
Total intracranial volume (in native space before scaling), intracranial-volume-corrected total gray matter (i.e., with linear effects of head size statistically removed), intracranial-volume-corrected total white matter, and intracranial-volume-corrected total CSF were assessed for group differences in two-sample t tests.
Next, SPM was conducted for the nine subjects with early-onset schizophrenia and the 10 comparison subjects by using SPM96. Three basic analyses were conducted to provide contrasts assessing the localization of differences between the two subject groups for 1) gray matter, 2) white matter, and 3) CSF. In all analyses, chronological age was used as a confounding covariate, and the height and extent thresholds required to identify regions of significant voxels were p=0.01 and 50 voxels, respectively.
In order to assess the validity and overall statistical significance of the SPM results for gray matter, white matter, and CSF, the subjects with early-onset schizophrenia and the comparison subjects were randomly assigned to groups
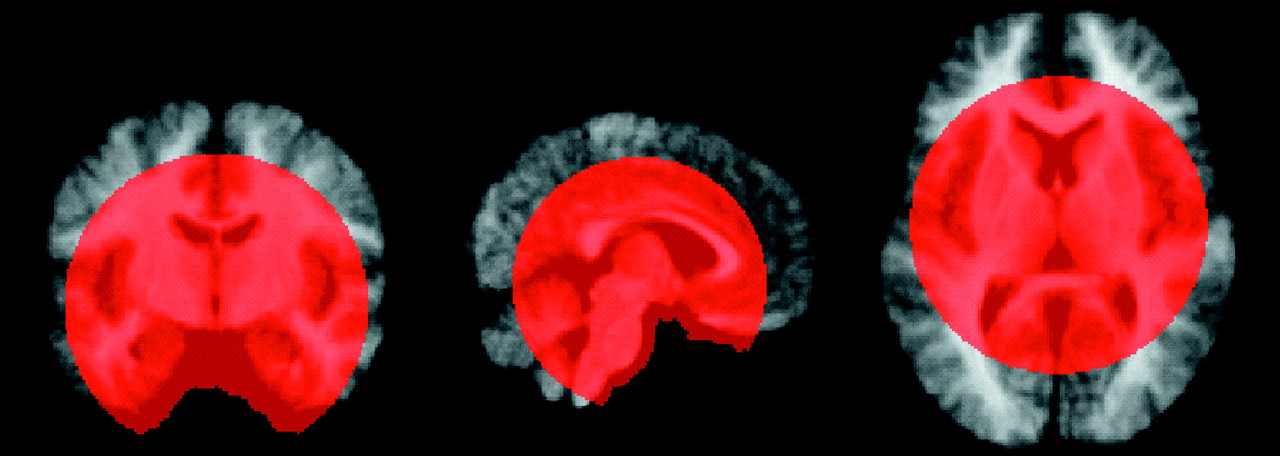
(38) for 30 new analyses (for each tissue type), from which the results (based on a more stringent height threshold, p=0.001) were tabulated. In these analyses, we counted the number of significant clusters revealed in the analyses of group differences (height threshold, p=0.001; extent threshold, 50 voxels) and compared that number to the number of significant clusters revealed in the randomized statistical parametric maps. Because we had a priori hypotheses regarding regional brain abnormalities, we created a gross volume of interest to encompass the subcortical region. The volume of interest was defined by creating a sphere with a radius of 60 mm with its center halfway between the midline decussations of the anterior and posterior commissures. The sphere encompassed approximately 35% of the total brain volume of one representative subject’s brain (in standard space) (
Figure 2). By excluding large brain regions about which we had no a priori hypotheses, we were able to make more careful decisions regarding statistical significance given the large number of voxel-by-voxel comparisons (approximately 1,350 11-mm smoothed voxels in the map for white matter). For the randomization test, we counted only clusters of 50 or more voxels within the volume of interest.
Volumetric analyses were conducted to test the regional hypotheses of abnormally large ventricles; group membership and age were used to predict regional ventricular volume (as repeated measures) in simultaneous multiple regression analyses. This analysis was designed to validate regional differences observed in the analyses of the SPM results. The lateral ventricles were defined on a slice-by-slice basis in each subject. The volumetric analyses were conducted in each subject’s native image space before scaling for importation into the statistical parametric maps. The ventricles were defined in the sagittal plane and included all voxels 1) that were classified as CSF and 2) that were within the frontal, occipital, or temporal horn or within the body of the lateral ventricles. Anterior regions (anterior to the midpoint between the anterior and posterior commissures) and posterior regions of the ventricles for each hemisphere were defined. The interrater reliability for ventricular volume was assessed as all 19 subjects’ ventricles were measured by two independent raters (E.R.S. and D.S.K.). The squared intraclass correlation coefficient was 0.992 for agreement on ventricular volume between the two raters, and the error estimate was 1%. Post hoc analyses were also conducted to detect between-group differences in the measures of the midsagittal area of the corpus callosum.
Discussion
The voxel-by-voxel analyses of gray matter, white matter, and CSF volumes revealed regional brain abnormalities in the early-onset schizophrenia subjects that were generally consistent with our a priori hypotheses based on prior studies. To our knowledge, abnormally large ventricles solely in the posterior region have not yet been reported for early-onset schizophrenia or childhood-onset schizophrenia. Other than large posterior ventricles, the brain abnormalities observed could result from tissue volumes that are actually smaller in the subjects with schizophrenia (in gray and white matter structures) or could result from local displacement of the structures due to the expansion of the lateral ventricles. Specifically, the callosal, cingulate, thalamic, and caudate abnormalities revealed in the SPM analyses, although not specifically predicted here, may be related to the large posterior ventricles given their spatial proximity. It is interesting that the area measurements of the corpus callosum did not differ between the two groups, suggesting that the location or shape, rather than the size, of this structure is abnormal in childhood-onset schizophrenia. Mesh-based parameterization was used in our laboratory
(39) to assess callosal shape variability in a group of patients with adult-onset schizophrenia, who were found to have an excess “bowing” of the corpus callosum. In that study, the curvature of the superior boundary of the corpus callosum was highly correlated with lateral ventricular volume. Perhaps we are observing a similar phenomenon in our group with early-onset schizophrenia.
The caudate and thalamic abnormalities observed in the SPM results for CSF difference maps also appear related to the large posterior ventricles given their location primarily in posterior regions. Here the group difference appears to result from voxels that are in the lateral ventricles in the schizophrenia group and in the posterior thalamus and internal capsule in the comparison subjects. Other research groups have reported thalamic abnormalities among patients with adult-onset schizophrenia in volumetric
(40) and voxel-based morphometry
(27) studies and among patients with childhood-onset schizophrenia
(18). Abnormally large basal ganglia have been reported in adults with schizophrenia
(16); this finding is likely related to neuroleptic exposure
(41). The caudate, putamen, and globus pallidus have also been found to be larger than normal in subjects with childhood-onset schizophrenia
(18,
19). Our SPM findings seem to suggest that basal ganglia and thalamic structures are smaller given that the regions with group differences are in CSF spaces in the schizophrenia group. However, it is possible that our results reflect displacement of these structures due to ventricular enlargement rather than truly smaller volumes.
Exposure to neuroleptics among patients with schizophrenia has repeatedly been shown to be related to enlargement of basal ganglia structures in adult-onset
(39,
40,
42) and childhood-onset
(43) schizophrenia. Given this, neuroleptic exposure in our subjects with early-onset schizophrenia must be taken into account in the interpretation of any neuroanatomical abnormalities observed in basal ganglia structures. We did not directly measure the volumes of these structures in our analyses, so we cannot rule out that abnormally large volumes are not present. Our results do provide evidence for displacement and perhaps for small caudate nuclei among the group with early-onset schizophrenia, which could be directly or indirectly related to the large ventricles. Thus, our findings are not consistent with effects that would be observed solely as a result of neuroleptic exposure.
The abnormalities in the posterior cingulate in the right hemisphere appear to result from regions that consistently are classified as white matter in the subjects with early-onset schizophrenia and gray matter in the comparison subjects. Benes
(44) reported that adults with schizophrenia had fewer neurons in the anterior cingulate cortex than their counterparts without schizophrenia. In addition, they found that the schizophrenia patients showed 25% more total vertical axons in this region than did comparison subjects. These results could account for our observation of relatively more white matter and apparently thinner gray matter in the cingulate region of the schizophrenia group. It is also possible that the large posterior ventricles are causing a shifting upward of the cingulate gyrus commensurate with the apparent upward “bowing” of the corpus callosum. In other words, the cingulate differences may be secondary effects rather than primary effects of lower cellular density in the group with early-onset schizophrenia.
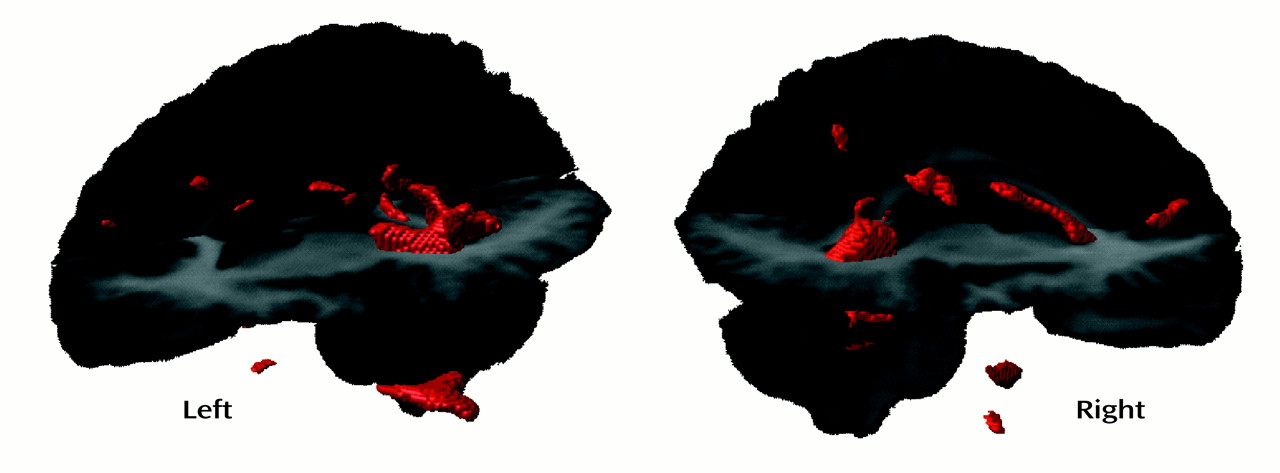
In the SPM results for white matter and CSF, the difference in posterior ventricular size appears to be confined to the left hemisphere, a finding that is consistent with findings for adult patients with schizophrenia, particularly men
(13). Our volumetric results, from analysis of data in native image space, indicate large ventricles primarily in the right hemisphere. The uncorrected SPM results for white matter (
Figure 5) reveal that even when assessed on a voxel-by-voxel basis, the difference in ventricular size is bilateral but is confined to the posterior horns and is perhaps more robust in the left hemisphere. The asymmetry ratios in
Table 2 revealed that the subjects with early-onset schizophrenia had relatively symmetrical ventricles, whereas the comparison subjects showed a left-larger-than-right asymmetry when the images were analyzed in native data space. The differences between the results from the volumetric and SPM analyses may reflect statistical power issues and may not actually be inconsistent. As shown in Figure 5, once the significance-level criteria were relaxed in the voxel-based analyses, bilateral differences in ventricular regions were observed. The results from the volumetric analyses revealed that the ventricles are larger bilaterally. To assume that the left is “more significant” than the right would be interpreting negative results that may be related to a lack of statistical power, rather than to any real anatomical difference between the groups. Thus, overall, we would not strongly interpret hemispheric differences in the posterior ventricular abnormalities given the present results.
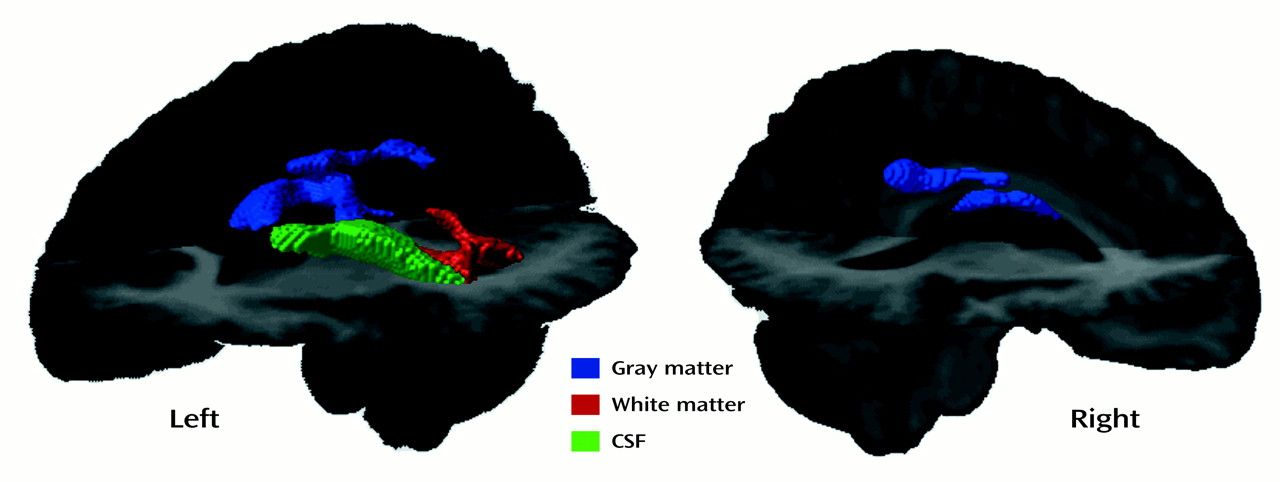
The etiology of the group differences we observed is likely related to a combination of factors. While we studied “gray” matter differences in one analysis and “white” matter differences in another, in addition to the “CSF” analyses, it is clear that the tissue types are not mutually exclusive in this kind of analysis given the smoothing and group averaging of the image data. Some of the differences appear to be due to voxels that are in fluid-filled regions in the subjects with early-onset schizophrenia and in white matter regions in the comparison subjects, such as in the case of the posterior ventricles and the corpus callosum. Other group differences resulted from voxels (such as in the cingulate region) that were categorized as gray matter in one group and as white matter in the other. The group differences revealed in the CSF analyses resulted both from shifts between gray matter and CSF (the caudate in the comparison subjects and ventricular CSF in the schizophrenia group) and shifts between white matter and CSF (ventricular CSF in the schizophrenia group and the posterior internal capsule in the comparison subjects). Thus, direct inferences about the type of tissue affected based on the type of binarized tissue maps entered into the SPM analyses cannot be made. Rather, the averaged brains of the schizophrenia and comparison groups (shown in
Figure 3) must be carefully inspected to determine the direction of the difference in tissue type between groups. These analyses suggest that we are likely seeing, directly or indirectly, the influence of robust enlargement in CSF spaces whether gray matter, white matter, or CSF statistical parametric maps are examined.
It is possible that the larger ventricles of our group with early-onset schizophrenia were a result of premature atrophy. The slightly smaller overall gray matter in the schizophrenia group supports this hypothesis. In addition, results from longitudinal studies of childhood-onset schizophrenia suggest progressive, perhaps degenerative, changes such as ventricular enlargement
(21) and progressive reduction of temporal lobe structures
(24) and cortical gray matter
(25). Rapoport and colleagues
(25) found that the lower gray matter volumes in childhood-onset schizophrenia were above and beyond those previously observed in cross-sectional studies of normal brain development conducted by other groups
(9,
10,
45–
47) and confirmed in her own longitudinal normal comparison group
(25). While the abnormality in gray matter volume observed in our subjects with early-onset schizophrenia was not nearly as robust as that reported by Rapoport and colleagues
(25), the patients with childhood-onset schizophrenia at the National Institute of Mental Health might have been more severely affected than our group with early-onset schizophrenia given that they, unlike our subjects, all had disorders refractory to typical neuroleptics.
In conclusion, the results from this study show that assessing structural brain abnormalities on a voxel-by-voxel basis can provide valuable insight into the neuromorphologic underpinnings of neuropsychiatric disorders. This combination of voxel-by-voxel and volumetric methods allowed us to verify what is arguably the most consistently reported abnormality in the schizophrenia literature, namely, large ventricles
(13). We also observed what may be more subtle effects in the cingulate, corpus callosum, thalamus, and caudate nucleus. These effects may be primary abnormalities or secondary effects related to the large ventricles. It should be noted, however, that these methods do not allow us to conclude that other regions of the brain are not also affected in early-onset schizophrenia. Rather, as we have shown here, the results from this type of analysis may be useful in highlighting regions that are of particular relevance to a specific disorder, such as early-onset schizophrenia, even when the neuromorphometric abnormalities are somewhat subtle.