Several studies have examined the plasma pharmacokinetics of selective serotonin reuptake inhibitors (SSRIs) in humans
(1). However, they did not measure either the concentrations or the elimination rates in the area where these compounds exert their therapeutic effect: the central nervous system (CNS). Determining the pharmacokinetics of such compounds in the brain is important for several reasons. First, no relationship between plasma concentrations of SSRIs and therapeutic response in depression has been identified
(1). Second, several SSRIs have been associated with the development of adverse effects following interruption or abrupt discontinuation of drug treatment
(2). The incidence of symptoms is thought to be related to rate of drug elimination, occurring more frequently in patients receiving drugs with faster clearance
(2). However, the relationship between CNS drug clearance and adverse effects has never been assessed.
We hypothesized that detecting a relationship between drug response and drug concentration could be facilitated by looking at the emergence of symptoms following abrupt discontinuation of some of the SSRIs. We tested this hypothesis in a subset of patients participating in a treatment-interruption protocol. The changes in fluorine magnetic resonance spectroscopy (MRS) signal in the brain of patients with remitted depression who were being treated chronically with one of two fluorinated SSRI medications (paroxetine, a monofluorinated phenylpiperidine, or fluoxetine, a trifluorinated propylamine derivative) were measured. In addition, the prevalence and severity of discontinuation symptoms were compared with the baseline brain and plasma drug concentrations, and the change in brain and plasma concentrations following abrupt discontinuation of drug under double-blind conditions was examined.
Method
This protocol was part of a larger study using magnetic resonance imaging (MRI) to assess changes in cerebral blood volume during different clinical states
(3). Patients meeting the criteria for unipolar depression, in remission, and taking 20 mg of either fluoxetine or paroxetine for 6 months to 3 years were recruited into this 6-week study. Patients were screened with a semistructured diagnostic interview that included the Structured Clinical Interview for DSM-III-R. Patients were excluded if they met the criteria for bipolar disorder, schizophrenia, or schizoaffective disorder or were actively abusing substances. They were also excluded if they met criteria for an axis II disorder, were taking centrally active medications (with the exception of one fluoxetine patient, who was taking 100 μg of thyroxine), or had a contraindication to MRI. The study was approved by the institutional review board of McLean Hospital, and all subjects provided written informed consent.
During the study, patients continued their own medications except for weeks 2 and 6, when they received blinded medications. The blinded medications contained 20 mg of active drug except on days 5 through 7 during 1 of the 2 study weeks, when they contained placebo. The order in which patients received placebo and drug was randomized. On days 4 and 7 of the 2 weeks in which patients received blinded medications, brain drug concentrations were assessed using 19fluorine MRS. This corresponded to 48–60 hours after the last dose of active medication during the placebo period.
During each of the two observation periods, blood was obtained for measurement of plasma drug concentrations, and we assessed the emergence of adverse events using an adverse events questionnaire
(2). The change in the number of events was determined by subtracting the number of new or worsened events reported during the preceding week, while taking active drug, from those reported during the observation period. Rating scales, attainment and analysis of the spectroscopic data, and measurement of serum drug levels were completed under double-blind conditions.
Oneida Research Services, Inc., using gas chromatography and mass spectrometry, determined serum fluoxetine and norfluoxetine concentrations. Paroxetine samples were analyzed by MEDTOX Laboratories, which used high-pressure liquid chromatography.
Spectroscopy data were acquired with a 1.5-tesla Signa scanner (General Electric, Milwaukee) and a quadrature volume head coil capable of being tuned to both the proton and fluorine resonance frequencies. Spectra were acquired by using a nonlocalized pulse acquisition method (TR=1 second, number of averages=1,000, total scan time=20 minutes). An external reference standard was placed beside the subject’s head to optimize data acquisition and normalize the brain signal. Structural scans were obtained for each patient during the study. The volume of each subject’s brain was estimated from the two-dimensional axial slices of the scans with Cine software
(4). Drug concentrations were determined by dividing the amount of drug in the brain by the brain volume.
Data analysis was performed by using Statview 5 (SAS Institute, Cary, N.C.). Statistical significance of differences and correlations was determined by using analysis of variance and Pearson’s correlation, respectively.
Results
Five subjects taking fluoxetine (one man and four women; mean age=31, SD=11) and five taking paroxetine (two men and three women; mean age=44, SD=16) underwent 19fluorine MRS to assess brain levels of fluorinated compounds on days 4 and 7 of weeks 2 and 6 of the study. Two patients were excluded from the final data analysis: for the first, a fluoxetine patient, the scanning was done according to a different signal acquisition protocol than the other patients, and for the second, a paroxetine patient, the spectra obtained during the placebo condition were uninterpretable because of signal artifact.
Following placebo substitution, a mean of 75% of the serum fluoxetine plus norfluoxetine remained (SD=13%), compared with a mean of 12% of the paroxetine (SD=2%) (F=96.75, df=1, 6, p<0.0001). In comparison, a mean of 88% of brain fluoxetine (plus fluorinated metabolites) signal (SD=13%) and a mean of 38% of the paroxetine (plus fluorinated metabolites) signal (SD=17%) remained (F=20.91, df=1, 6, p=0.004). After substitution with active drug, the change in fluorine signal did not differ significantly between the two drugs (F=0.12, df=1, 5, p=0.75).
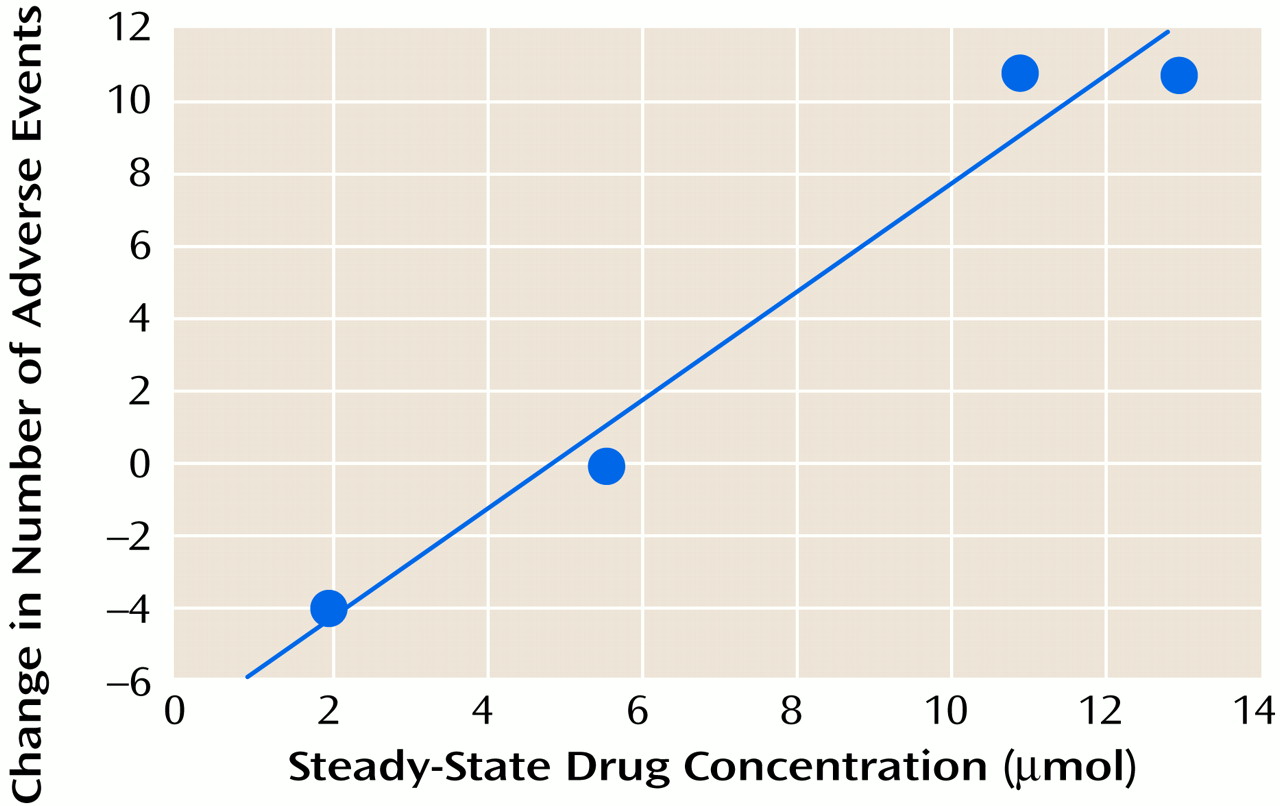
Among patients taking paroxetine, the increase in reported adverse events during the placebo condition correlated significantly (Pearson’s correlation) (
Figure 1) with the brain drug level before substitution with placebo. A corresponding relationship was not observed for the fluoxetine patients (r=0.25, df=2, p=0.75). It is also of note that despite the fact that each patient was taking 20 mg/day of drug, the brain levels at steady-state varied approximately sevenfold across patients for each medication.
Discussion
Higher steady-state brain levels of paroxetine were associated with greater risk for adverse events after interruption of treatment. The decrease in brain concentration for each drug was similar to, but smaller than, the decrease in serum concentration. Thus, brain elimination appeared to be slower than serum elimination.
The differences in the amount of drug cleared from the serum and the brain observed here may reflect the differences between clearance in a peripheral compartment (serum) and a deep compartment (the brain) or differences between a more aqueous (serum) and a more lipid-rich (brain) compartment. However, they may also reflect differences in assay methodologies. 19Fluorine MRS measured total fluorine signal, which includes the parent drug and all fluorinated metabolites in the brain, but the serum drug assays measured only the parent drug compounds and norfluoxetine.
The two drugs were not evaluated after comparable decreases in drug levels, which limited our chances of finding discontinuation-associated adverse events in the fluoxetine group. However, we studied the common clinical scenario of patients missing two to three doses of medication. Although the small number of patients studied limits the interpretation of the data, the findings suggest that brain drug levels correlate with clinical effect and merit further investigation.