Persistent insomnia, defined as problems initiating and/or maintaining sleep at least three nights/week accompanied by daytime distress or impairment (ICD-10), is associated with an array of individual and societal consequences, including greater medical and psychiatric morbidity
(1–
7), life-threatening accidents, reduced quality of life, impaired job performance, and absenteeism
(3,
8–
12). Ten percent to 15% of adults report persistent sleep problems
(13–
17); the rates of sleep problems among women and older adults are even higher
(18–
21).
The cost of insomnia in terms of lost productivity and accidents has been estimated to be $77–$92 billion annually
(22). Despite these costs, the overwhelming majority of individuals with insomnia remain untreated
(17). More than 50% of primary care patients experience insomnia
(13), but only about one-third mention this problem to their physicians
(23), and only 5% seek treatment
(13). Most patients with insomnia (67%) report a poor understanding of treatment options, and many turn to alcohol (28%) or untested over-the-counter remedies (23%)
(13). This is particularly unfortunate given that insomnia can be readily diagnosed and treated. Four meta-analyses
(24–
27), two pharmacological and two behavioral, summarized more than 150 controlled investigations supporting the efficacy of treatments for primary insomnia.
The meta-analyses of pharmacotherapy support short-term (2–4-week) effectiveness of medication compared with placebo. Benzodiazepine receptor agonists like temazepam, zolpidem, and zaleplon were the most widely used medications. Clinical gains were reported to be very reasonable
(24), with preferential effects on total sleep time
(25). Perhaps the primary limitation of pharmacotherapy is the absence of data regarding long-term efficacy. Long-term use has been thought to result in tolerance, dependence, and rebound insomnia on discontinuation
(28–
30). Limited evidence from two uncontrolled open-label studies with zolpidem and zaleplon, however, indicates that these medications may be effective for 3 to 6 months without dose escalation
(31,
32). No data suggest sustained improvement when medication is withdrawn.
Two meta-analyses support behavioral interventions for improving sleep
(26,
27). Behavioral treatments focus on modifying contingencies thought to maintain chronic insomnia
(33). Effective treatment typically involves four to eight weekly sessions and requires substantial patient motivation. The most efficacious components are considered to be stimulus control and sleep restriction
(26). Sleep hygiene instructions and cognitive therapy may be included as well. Advantages of behavior therapy are minimal side effects and sustained improvement. Treatment gains have been documented from 6 months to 2 years
(27,
34,
35). Primary limitations for behavioral treatment include 1) a shortage of trained specialists, 2) cost and variable insurance reimbursement, and 3) the assumption that medications are more efficacious.
Only two experimental studies directly compared behavioral and pharmacological treatments
(34,
36). Both investigations found 1) comparable treatment effects, 2) more rapid improvement with sedative hypnotics, and 3) more sustained improvement with behavioral treatments. Systematic documentation of the relative efficacy of these treatments is needed. Unfortunately, directly comparing the effect sizes from the behavioral
(26,
27) and pharmacological
(24,
25) meta-analyses is not possible because of a variety of factors that make the literature on the outcome of the two treatments dissimilar. These factors include 1) incompatible study designs, 2) different outcome measures, and 3) inconsistent criteria for the definition of insomnia.
The objective of the present study was to evaluate pharmacological and behavioral treatments by using criteria that maximize fair comparisons of the literature on the outcome of pharmacotherapy and behavior therapy.
Method
Data Sources and Study Selection
Published studies were identified by using the keywords “insomnia” and “treatment” in English-language searches of MEDLINE and psycINFO databases from 1966 to 2000 and from bibliographies provided by the authors of two meta-analyses of insomnia
(24,
26).
Our intent was to identify a broad range of studies that used definitions of primary insomnia that were consistent with current definitions and that used treatments considered most effective for insomnia: 1) benzodiazepines and benzodiazepine receptor agonists (e.g., zolpidem, zaleplon, or zopiclone) and 2) stimulus control and sleep restriction. We included only studies that reported sleep continuity measures in minutes and were based on mean values before and after treatment derived from prospective, self-report sleep diaries. Diary data were chosen because this measurement strategy is most frequently used and because it represents the primary means of assessing patients in practice
(24,
26,
27). Within-subject designs were chosen to provide estimates of improvement from before treatment.
Inclusion Criteria
1. The investigation was a treatment study for primary insomnia.
2. Duration of insomnia was 1 month or longer.
3. Sleep diary measures were reported.
4. Psychiatric and general medical conditions were excluded.
5. Pharmacological studies included benzodiazepines or benzodiazepine receptor agonists (zolpidem, zopiclone, zaleplon).
6. Behavior treatments included stimulus control or sleep restriction.
7. Within-subject measurements were obtained before and after treatment.
Exclusion Criteria
1. Sleep continuity variables were presented as ordinal data.
2. No means or standard deviations were presented.
3. Patients were not withdrawn from hypnotic medications before the trial.
Outcome Variables
In the present analysis we reviewed and evaluated three sleep continuity variables: sleep latency, total sleep time, and number of awakenings. These variables were also evaluated in the previous meta-analyses
(24–
27). Additionally, we provide information from the literature on the outcome of the two treatments on subjective sleep quality and wake time after sleep onset. Very few of the pharmacological studies included a measure of wake time after sleep onset. We include the measure here because of its clinical relevance, but we recognize that these findings may not be representative.
Calculation of Effect Sizes and Data Extraction
A measure of effect size was the primary indicator of treatment outcome. Effect size provides a measure of change in standard deviation units (i.e., magnitude of response relative to variability). A standardized mean difference score was calculated for each outcome variable by using Cohen’s d index of an individual effect size (di=[M1–M2]/SDpi)
(37), where
d=effect size,
i=individual study,
M1=pretreatment mean,
M2=posttreatment mean, and
SDp=pooled standard deviation. The pooled standard deviation was calculated by summing the reported pretreatment and posttreatment standard deviations and dividing by 2. In the rare instance where both standard deviations were not reported but means and the t statistic were available, the pooled standard deviation was calculated according to the formula (t=M1–M2/SDpi*square root of [(1/N1)+(1/N2)])
(38). When the standard error of the mean was provided, the standard deviation was calculated according to the formula (SD=SEM*square root of N)
(38). Individual effect sizes were weighted to account for individual sample sizes. The overall weighted effect size was calculated according to the formula (Σ[di*Ni]/Σ[Ni])
(39).
All studies were reviewed and coded by two of us (M.T.S. and A.P.) to determine whether inclusion and exclusion criteria were satisfied. Studies were coded to extract major clinical variables, including demographics, type and duration of treatment, and the outcome variables. One of us (M.L.P.) resolved discrepancies between ratings of each study. All values entered into the final database were verified by a research assistant.
Results
Excluded Studies
We identified 194 treatment outcome studies of primary insomnia of more than 1 month’s duration. Almost half (N=85; 44%) were excluded because pretreatment-posttreatment effect sizes could not be calculated because of parallel design or because means, standard deviations, or an F or t statistic were not reported. Twenty-five percent (N=49) were excluded because prospective sleep diaries were not used. Thirteen percent (N=26) were ineligible because sleep continuity variables were presented as ordinal data. Eight behavioral studies (4%) were excluded because stimulus control or sleep restriction were not used. Five studies (3%) were ruled out because subjects were not withdrawn from hypnotic medications before the trial. Twenty-one studies satisfied criteria for meta-analysis
(34,
35,
40–
58).
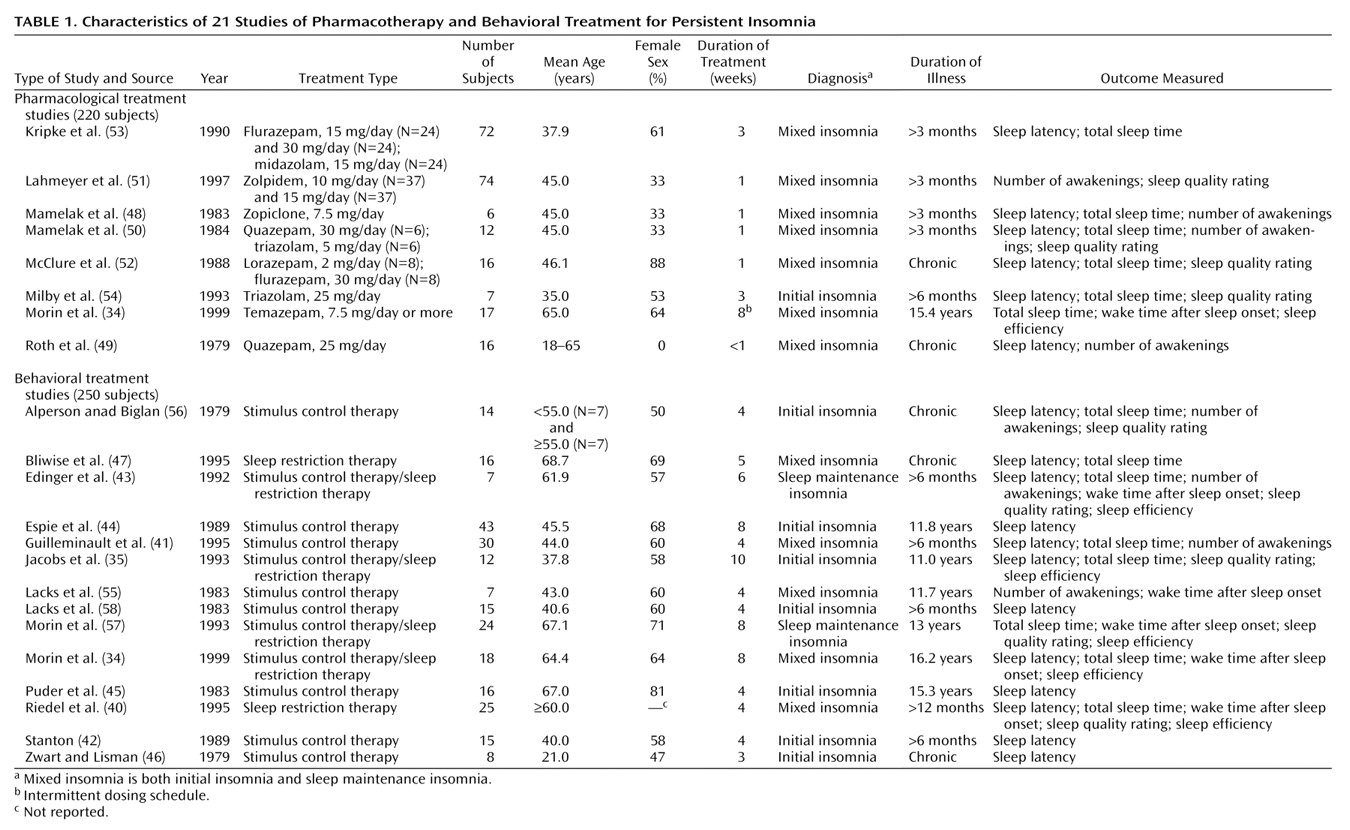
Characteristics of Selected Studies
Studies spanned the years from 1979 through 1999 and summarized outcomes for 470 subjects.
Table 1 displays the clinical characteristics of subjects by type of treatment. Seven investigations
(48–
54) evaluated only pharmacological therapies (203 subjects), 13 studies
(35,
40–
47,
55–
58) evaluated only behavioral interventions (232 subjects), and one study
(34) compared pharmacotherapy with behavioral therapy (N=35). Most studies involved multiple groups and included both sexes (246 [55%] of the 445 subjects in studies reporting gender were female). Participants were middle-aged (mean=47.2 years, SD=11) and had diagnoses of mixed insomnia (trouble initiating and maintaining sleep) of 3 or more months’ duration.
Seven pharmacotherapies were represented; the mean length of treatment was approximately 2 weeks (SD=2). Flurazepam was used in three groups. Quazepam, triazolam, and zolpidem were each used in two groups. Lorazepam, midazolam, and zopiclone were each used in one group.
Twelve of the behavioral studies included stimulus control therapy with or without sleep restriction; two studies used sleep restriction alone; and four combined stimulus control and sleep restriction. The mean number of behavior therapy sessions was five (SD=2) over a mean period of approximately 5 weeks (SD=2).
There were no differences in subjects’ sex, age, and pretreatment means for sleep latency, number of awakenings, wake time after sleep onset, total sleep time, and subjective sleep quality between pharmacotherapy and behavioral treatment (p>0.05). As expected, duration of behavioral treatment was significantly longer than pharmacotherapy (t=–4.38, df=26.49, p<0.001). These comparisons indicate that the two treatment populations were similar.
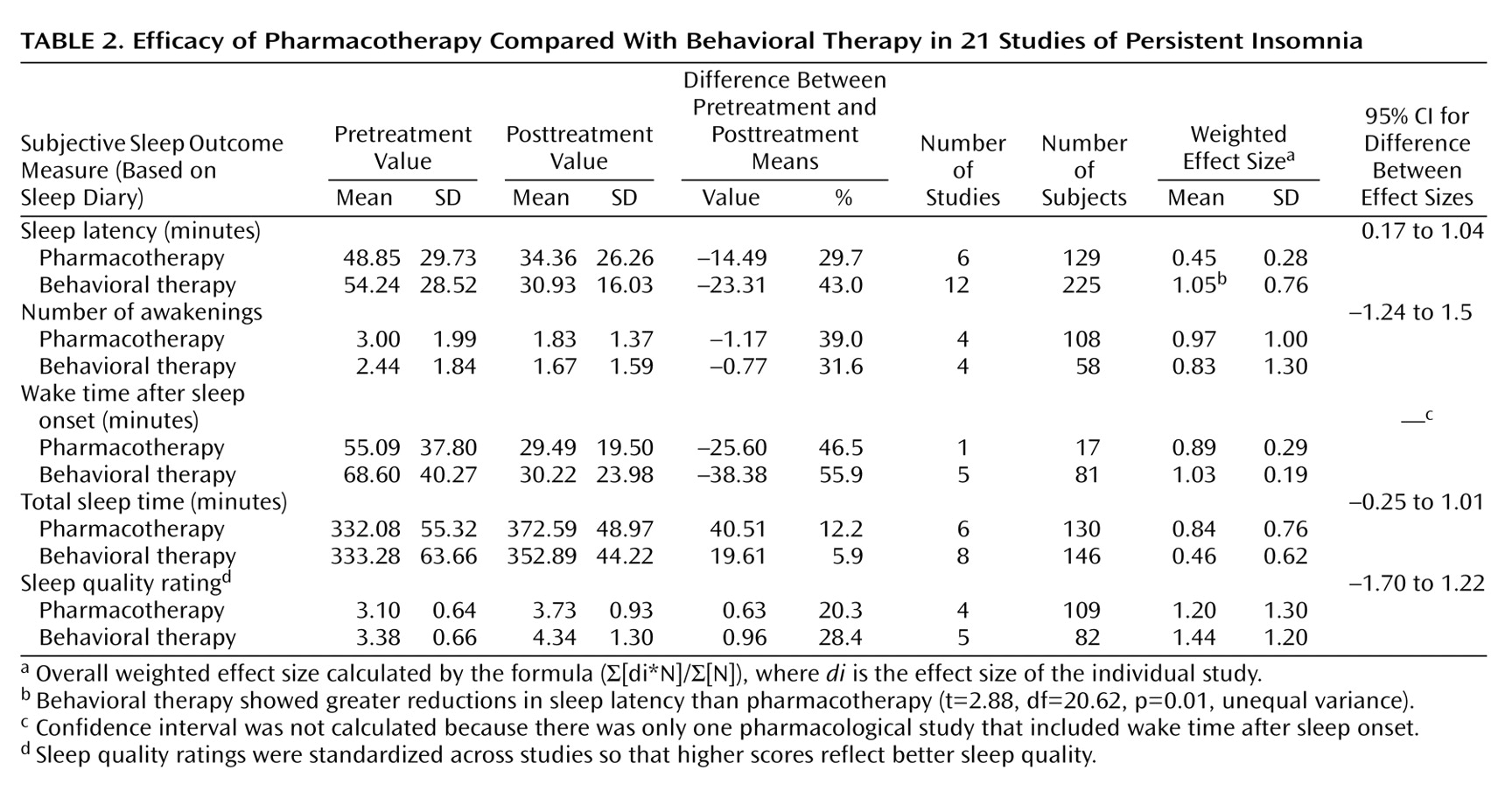
Comparison of Treatment Effects
Table 2 presents the mean values before and after treatment and the overall weighted effect sizes for five major outcome variables. Sleep latency was reduced by 30% with pharmacological treatment, compared with 43% with behavioral interventions. Both treatments reduced number of awakenings each night by approximately 1. Wake time after sleep onset was reduced by 46% with pharmacotherapy and 56% with behavior therapy. Both interventions demonstrated moderate improvement in total sleep time. Pharmacotherapy increased total sleep time by 12% and behavior therapy by 6%. Pharmacotherapy improved sleep quality by 20% and behavior therapy improved sleep quality by 28%.
The mean effect size for all five outcome variables was 0.87 for pharmacotherapy and 0.96 for behavior therapy, suggesting comparable efficacy in improving sleep continuity and sleep quality at the end of acute treatment. For the five individual sleep variables, independent t tests for unequal variances were calculated to compare the weighted effect sizes for pharmacotherapy and behavioral therapy. Only the effect sizes for sleep latency significantly differed (
Table 2).
Levine’s test for equality of variances was calculated to evaluate whether either treatment demonstrated greater variability of effect sizes for the outcome variables. Behavioral therapy had greater variability in weighted effect sizes for sleep latency than pharmacological studies (F=8.05, df=20.62, p=0.01).
Discussion
We compared the acute effects of pharmacotherapy and behavioral therapy for primary insomnia by conducting a meta-analysis of outcome studies. Both treatments yielded similar results overall. The mean effect size for both treatments was greater than 0.80, indicating a large treatment effect
(37). The majority of weighted individual effect sizes were large and were comparable across treatments. The exceptions were sleep latency and total sleep time. For sleep latency, behavioral treatment produced significantly larger treatment effects. For total sleep time, the effect size corresponding to the behavioral intervention was moderate, but it was not different from the effect size of pharmacotherapy.
Three caveats must be considered before any firm conclusions may be drawn. First, the mean difference between the two effect sizes for sleep latency was estimated to range from 0.17 to 1.04 (95% confidence interval). This range indicates that the true difference between these effect sizes may be relatively modest; therefore, it is not clear that behavioral therapy represents the better choice for sleep initiation problems. Second, with behavioral treatment, there was greater variability in effect sizes for sleep latency, suggesting less reliability in delivering a consistent treatment effect. Third, it is possible that the modest finding for sleep latency with benzodiazepine receptor agonists may be more related to experimental design considerations than the potency of hypnotics. Most of the studies evaluated agents with long latencies to peak plasma concentration (e.g., for lorazepam the latency is 120 minutes) and provided the drug at bedtime. Thus, the maximal sedative effect would be more likely to influence sleep maintenance than sleep initiation.
Given these caveats, it remains possible that behavior therapy is more effective for sleep initiation problems. This may be because the manipulation of factors related to the homeostatic regulation of sleep (by means of sleep restriction) may elicit sleep more potently than the pharmacological manipulation of γ-aminobutyric acid neurotransmission. The mechanism(s) of action for behavior therapy might also account for the only moderate
short-term effects of behavioral treatment on total sleep time
(34). Behavior therapies initially curtail sleep opportunity to increase the homeostatic drive for sleep. Sleep opportunity is increased systematically only when sleep is consolidated. Thus, behavioral interventions are not particularly efficacious in increasing total sleep time in the short-term. Perhaps the more important question is, What happens long-term? One meta-analysis
(26) indicated that total sleep time continues to increase beyond short-term treatment levels with behavior therapy.
Limitations
Differences between behavioral and pharmacological outcome studies
The most obvious difference between the literature on the outcome of behavioral and pharmacological treatment is related to the number of contacts patients have with their clinicians. Thus, it might be argued that comparable efficacy is due to greater patient access to clinician support with behavioral treatment. Whether such contact would augment pharmacotherapy and lead to superior short-term outcomes is an empirical question.
Self-selected samples
Absence of random assignment is a primary limitation of any comparative meta-analysis. The most appropriate conclusion that can be drawn is that, under optimal conditions, in which patients choose their method of treatment, the behavioral and pharmacotherapeutic treatments yield equivalent outcomes. It cannot be concluded that the treatments yield comparable gains across all patients. In the clinical arena, however, patients are not randomly assigned; they choose treatments that are personally preferable. One might argue, therefore, that although our scientific generalizability is inherently limited, our generalizability to the clinical setting is enhanced.
Subjective versus objective measures of outcome
Prospective sleep diaries are by far the most consistently used measure of sleep in the studies of both pharmacological and behavioral treatment and in clinical practice. Thus, the present quantitative review reflects the state of the literature on the outcome of the two modes of treatment. It should be noted, however, that patients with insomnia routinely overestimate sleep latency as well as wake time after sleep onset and underestimate total sleep time compared with polysomnography measurement. Although polysomnography in combination with sleep diaries provides a better evaluation of sleep, subjective complaints are necessary and sufficient to make the diagnosis of primary insomnia. Diaries have also been found to be highly correlated with polysomnography-defined sleep continuity
(59) and sensitive to polysomnography-defined changes in sleep
(60).
Implications
The present study extends previous meta-analytic work
(24–
27) by demonstrating that behavior therapy for persistent primary insomnia is as effective in the short-term as pharmacotherapy. The question, then, is which treatment modality should be used. Pharmacotherapies may be selected when immediate symptom reduction is the primary consideration. Behavioral treatment may be indicated when pharmacotherapies are contraindicated (e.g., because of potential drug interactions or history of substance abuse). Ultimately, however, the difference in treatment cost is likely to be a major consideration. Even the most expensive sedative hypnotics, in the short run, do not rival the costs of behavior therapy. For example, a 35-day trial of zolpidem or zaleplon costs approximately $166 (30-minute office visit with primary care practitioner at $75.00; 35 tablets at $2.60/10 mg tablet), compared with $350 for 5 weeks of behavioral treatment ($100 for 50-minute session multiplied by 2; $50 for 30-minute sessions multiplied by 3). Cost accounting, however, must also consider the consequences of discontinued treatment and the possibility that the long-term benefits of behavioral treatment might offset the short-term premium by reducing indirect costs (e.g., absenteeism, lost productivity, psychiatric morbidity). More research is required before an overall cost-benefit analysis can be conducted.
Finally, cost issues aside, many patients and clinicians do not wish to use hypnotics to treat persistent insomnia. In these instances, practitioners should strongly consider referring patients for behavior therapy, and some should consider training in behavioral treatment.