Severe premenstrual syndrome (premenstrual dysphoric disorder in DSM-IV) consists of affective and somatic symptoms that appear during the luteal phase of the menstrual cycle and remit shortly after the onset of menses. Although the etiology of premenstrual dysphoric disorder has not been established, the potential role of the serotonergic (5-HT) system is suggested by the following (reviewed by Rubinow and Schmidt
[1]): 1) the acute improvement of premenstrual syndrome symptoms after administration of
m-chlorophenylpiperazine (
m-CPP), a mixed 5-HT agonist/antagonist; 2) diagnosis-related differences found in peripheral measures of serotonin between women with premenstrual dysphoric disorder and comparison women (in some studies, but not others); 3) the role of serotonin in many of the symptoms and behaviors characteristic of premenstrual syndrome (e.g., appetite dysregulation); and 4) importantly, the alleviation of premenstrual dysphoric disorder symptoms with serotonergic agents such as fluoxetine.
It is unknown whether women with premenstrual dysphoric disorder have an underlying dysregulation in serotonergic neurotransmission that is compensated for by treatment with selective serotonin reuptake inhibitors (SSRIs) or whether the change in serotonin with SSRIs is therapeutic but unrelated to the pathophysiology of premenstrual dysphoric disorder. It is also unknown whether SSRIs such as fluoxetine have a different mechanism of action in premenstrual dysphoric disorder compared with major depression and obsessive-compulsive disorder (OCD), two other disorders responsive to these agents. Unlike these last two disorders, symptoms in premenstrual dysphoric disorder have been reported to resolve with acute, short-term use of SSRIs (e.g., dosing only during the luteal phase)
(2). SSRIs, in general, are thought to exert their effects through adaptive changes consequent to their inhibition of the serotonin transporter
(3). However, changes due to direct binding of the receptors cannot be ruled out, since it has been demonstrated that fluoxetine directly binds to the 2C receptor
(4). Further, other potential mechanisms may exist. Data suggest that SSRIs rapidly increase the activity of the steroidogenic enzyme 3-alpha hydroxysteroid oxidoreductase, which is involved in the synthesis of neurosteroids (e.g., allopregnanolone) etiologically implicated in both depression and premenstrual syndrome
(5).
One means of exploring the role of the serotonin system in women with premenstrual dysphoric disorder is the inhibition of serotonergic function with selective receptor antagonists. Antagonists specific for individual receptor subtypes have been largely unavailable for use in humans, but some serotonin system-specific antagonists are available. Metergoline is a serotonin-selective antagonist that effectively blocks most 5-HT receptor subtypes at a relatively low dose. Its highest affinity is for 5-HT
2A and 5-HT
2C receptors
(6). At low doses, it does not have effects on the norepinephrine, acetylcholine, GABA, or dopamine neurotransmitter systems
(7) and has been shown to be relatively well tolerated
(8).
Short-term blockade of serotonin receptors additionally may be of benefit in disentangling the acute and chronic consequences of serotonergic modulation. Indeed, the complexity of the serotonin system is such that one cannot infer the direction of the effect of serotonin modulation on the basis of therapeutic efficacy of SSRIs. For example, both OCD and premenstrual dysphoric disorder respond to SSRIs, but while the serotonin agonist
m-CPP decreases symptoms in premenstrual dysphoric disorder, it increases symptoms in OCD
(9,
10). Additionally, tryptophan depletion does not reverse the efficacy of SSRIs for the characteristic symptoms of OCD (although mood deteriorates)
(11), whereas it increases symptoms in premenstrual dysphoric disorder
(12). In order to assess the role of acute serotonergic modulation in the efficacy of SSRIs in women with premenstrual dysphoric disorder, we administered metergoline in a double-blind, placebo-controlled study to 1) patients with premenstrual dysphoric disorder who had experienced symptom remission during treatment with fluoxetine and 2) a group of unmedicated healthy comparison women (to ensure that any symptoms induced were not nonspecific effects of study medications).
Method
Subject Selection
Women with severe premenstrual syndrome/premenstrual dysphoric disorder were either self-referred in response to advertisements in local newspapers or referred by their physician. Comparison women were recruited through local advertisements. All subjects were without current medical illness (as determined by a medical history review, physical examination, laboratory tests, and an ECG), were medication free, and had no axis I diagnosis within the past 2 years, including alcohol and substance abuse, as determined by the Structured Clinical Interview for DSM-III-R or DSM-IV (SCID)
(13,
14). Premenstrual syndrome was confirmed by prospective ratings for three consecutive cycles. In order to be confirmed as having a diagnosis of premenstrual syndrome, subjects had to show at least a 30% increase in mean negative mood (e.g., depression, anxiety, or irritability) on a 100-mm analogue rating scale in the week before menses compared with the week after menses (unpublished guidelines from the NIMH Premenstrual Syndrome Workshop, Rockville, Md., 1983). Patients also met criteria for DSM-IV premenstrual dysphoric disorder.
A group of unmedicated healthy comparison women participated as well in order to control for symptoms that might be secondary to side effects of the metergoline. Comparison women also completed 2 months of prospective ratings, were without premenstrual mood symptoms, and did not meet criteria for either current or past psychiatric illness as determined by the SCID.
All subjects had regular menses, ranging between 21 and 34 days in length. Pregnancy tests were performed before the beginning of the study, and all participants were required to use barrier forms of contraception throughout the course of the study. The protocol was reviewed and approved by the NIMH Institutional Review Board, and all subjects gave both written and verbal consent to study participation. Since metergoline has been shown in other patient populations to produce clinically significant worsening, for ethical reasons patients who had been suicidal within the last year were excluded from participation. None of the women with premenstrual dysphoric disorder had any history of psychiatric hospitalization.
Medications
Metergoline, fluoxetine, and diphenhydramine were purchased from commercial sources. Metergoline (Farmitalia Carlo Erba SRL, Milan, Italy) was analyzed by the National Institutes of Health Pharmaceutical Development Service (Bethesda, Md.) to verify its identity and purity. The 8-mg dose was selected on the basis of previous studies
(8) that demonstrated that this dose produced behavioral effects in selected populations and was generally well tolerated.
Procedure
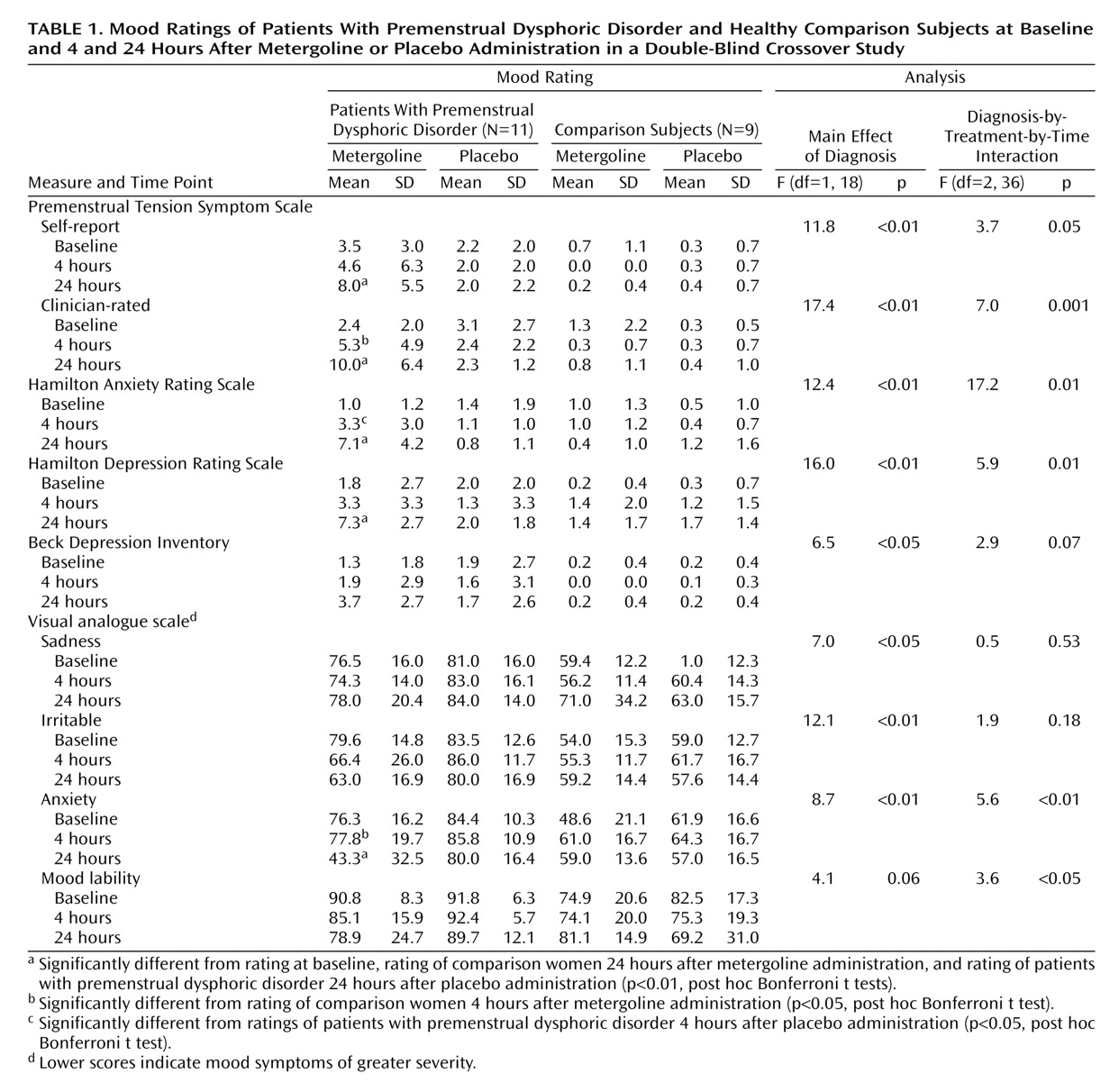
After acceptance into the study, participants with premenstrual dysphoric disorder (N=13) were given a trial of open fluoxetine for a period of 3 months. We used a flexible-dosing schedule titrated to symptom relief (doses ranged from 7.5 mg/day to 40 mg/day). Subjects who exhibited a response to fluoxetine (i.e., failed to meet the aforementioned 30% increase in mean negative mood) (N=11) then participated in a randomized, double-blind, placebo-controlled, crossover study 6–8 days after their luteinizing hormone surge during two consecutive menstrual cycles as determined by Ovuquick test kits (Quidel Corp., San Diego). The timing and nature of the procedure were identical in the unmedicated healthy comparison group (N=9).
Participants came to the clinic between 8:00 a.m. and 9:00 a.m. after fasting from midnight the night before. After baseline vital signs and mood ratings (Beck Depression Inventory
[15], self-report and clinician-rated forms of the Premenstrual Tension Symptom Scale
[16], Hamilton Anxiety Rating Scale
[17], Hamilton Depression Rating Scale
[18], a measure of side effects
[19], and a visual analogue scale [
20,
21]) were obtained, a heparin lock was inserted for blood drawing purposes. Metergoline (8 mg) or diphenhydramine (50 mg) was administered orally 40 minutes after insertion of the heparin lock. Diphenhydramine was used as an active placebo to maintain the blind and to control for the sedative effects of the metergoline. Baseline blood samples were obtained at –20 and –5 minutes and then at 30, 60, 90, 120, 180, and 240 minutes after administration of the medication. Mood ratings were obtained at baseline, at the end of the procedure (4 hours after administration of medication), and 24 hours after the procedure.
Physiologic Measures
Vital signs (temperature, pulse, and blood pressure) were obtained at baseline and after 30, 60, 90, 120, 180, and 240 minutes.
Blood samples were collected for the measurement of plasma levels of prolactin
(22) (a measure of the central activity of metergoline) at baseline and 30, 60, 90, 120, 180, and 240 minutes (4 hours) after metergoline/active placebo administration. Metergoline levels were obtained at baseline and 60 minutes, 4 hours, and 24 hours after metergoline administration. Additionally, patients had fluoxetine and norfluoxetine levels drawn at the end of the procedure (i.e., after 4 hours) and after 24 hours.
Plasma prolactin assays were performed at Covance Laboratories (Vienna, Va.) by methods previously described
(23). The prolactin assay had an intraassay coefficient of variation of 4.6% and an interassay coefficient of variation of 8.2%. Fluoxetine and norfluoxetine assays were performed by high-performance liquid chromatography by Quest Diagnostics (Baltimore). Metergoline levels were assayed by a high-performance liquid chromatography method developed in the Laboratory of Clinical Sciences, NIMH (Bethesda, Md.) (T. Toliver et al., personal communication). The intraassay and interassay coefficients of variation were 5.8% and 8.8%, respectively.
Symptom Ratings
Participants completed the following symptom ratings at baseline, at the end of the procedure (4 hours), and at 24 hours: the Beck Depression Inventory
(15), self-report Premenstrual Tension Symptom Scale
(16), and a physical symptoms rating scale
(19). Visual analogue scales (100-mm) of mood measures—mood stability, irritability, sadness, and anxiety—were also completed by the subjects at the same time points. In addition, the following objective measures were obtained (at the same time points mentioned for the self-ratings): the Hamilton depression and anxiety scales
(18) and the clinician-rated Premenstrual Tension Symptom Scale
(16).
Statistical Analysis
Symptom ratings were analyzed by repeated-measures analysis of variance (ANOVA) with Systat 8 (SPSS, Chicago, 1998), with drug treatment (metergoline versus diphenhydramine) and time (baseline, 4 hours [postprocedure], and 24 hours) as the within-group variables and diagnosis the between-group variable. Significant differences in these measures were analyzed with post hoc Bonferroni t tests. Pearson correlation coefficients were calculated to determine if symptoms at 4 and 24 hours after the procedure were predicted either 1) by plasma levels of fluoxetine or norfluoxetine at the end of the procedure or after 24 hours or 2) by metergoline levels at 60 minutes (peak) and at the end of the procedure (metergoline levels at 24 hours were negligible).
Discussion
A variety of convergent data suggest that the central serotonergic systems play a major role in the pathophysiology and treatment of premenstrual syndrome. These data include, but are not limited to, observations that serotonergic agonists are effective in ameliorating premenstrual dysphoric disorder symptoms. Nonetheless, several observations raise questions about the means by which SSRIs alleviate the symptoms of premenstrual dysphoric disorder. First, the time course of the effect of SSRIs is very rapid, ostensibly requiring only a few days for efficacy, an observation that has led to the successful luteal phase-only administration of SSRIs
(24). These rapid effects suggest that SSRIs may be acting through a mechanism that is distinct from that in depression, which characteristically requires a longer latency until expression of antidepressant effects. Second, in keeping with the rapidity of therapeutic response to SSRIs, their rapid induction of neurosteroid synthesis would be expected to alter GABAergic signaling and thus provides an alternative psychotropic mechanism
(5,
25). Hence, it is not clear that the symptom mitigating effects of SSRIs in premenstrual syndrome are mediated through typical serotonergic modulatory actions.
In this study, we have demonstrated that administration of the serotonin receptor antagonist metergoline precipitates a significant symptomatic relapse in euthymic women with premenstrual syndrome treated with fluoxetine. The significant precipitate mood symptoms (particularly irritability, anxiety, and tension [data not shown]) appeared primarily 24 hours after the procedure, a time when precipitate symptoms also appeared in OCD patients receiving fluoxetine
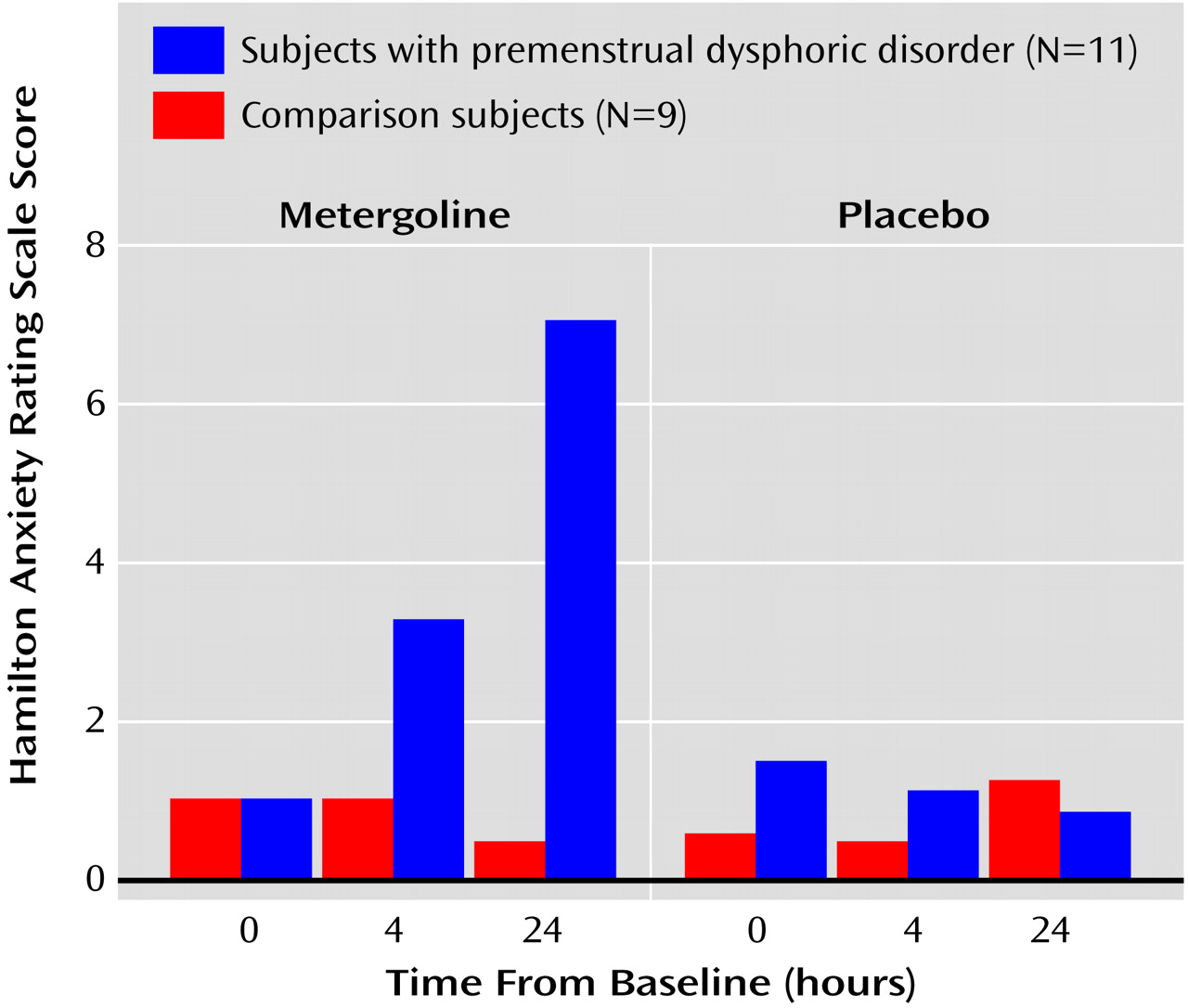
(8). A slight but significant increase in symptoms was observed in patients after 4 hours (the Hamilton anxiety scale compared with baseline; clinician-rated Premenstrual Tension Symptom Scale relative to comparison women). Patient mood ratings did not differ from comparison subjects at baseline or at the end of the procedure, and neither patients nor comparison women experienced significant symptom precipitation at any time after the active placebo.
The delay in the appearance of symptoms after metergoline administration is of interest for several reasons. First, this time course is compatible with the development of some of the known effects of metergoline. For example, preclinical data have demonstrated that metergoline can produce 5-HT receptor downregulation that can last for several days
(26), and there is some evidence that some of the actions of the serotonin antagonists occur in a delayed manner (e.g., decrease in 5-HT
1D receptor density in rat hippocampus 24 hours, but not 1 hour, after administration of a 5-HT antagonist)
(27). This raises the possibility that these serotonin subsystems may convey some of the efficacy of SSRIs in premenstrual dysphoric disorder. Second, the latency to symptom onset is longer than that observed following tryptophan depletion; one report describes the reversal of the efficacy of SSRIs in premenstrual syndrome 10 hours, but not 24 hours, after the tryptophan depletion procedure
(12). While these different kinetics may implicate different serotonin receptor subtypes, the tryptophan depletion procedure pre-depletes the subjects for 1 day before the test, suggesting that a similar delayed effect may characterize both methods of decreasing serotonergic activity. Alternatively, the significant increase from baseline in the Hamilton anxiety scale scores after 4 hours in the women with premenstrual dysphoric disorder (and the increase in clinician-rated Premenstrual Tension Symptom Scale scores relative to comparison women) suggests that at least some symptom emergence occurs fairly acutely, compatible with the time course described with tryptophan depletion. Both the study by Menkes et al.
(12) and our data do suggest the role of altered serotonergic neurotransmission in the efficacy of the SSRIs in premenstrual dysphoric disorder. This inference is further strengthened by our observation that metergoline levels at 60 minutes and 4 hours were significantly correlated with the severity of the symptoms experienced at 24 hours in the patient group. Further, our study is not complicated by the appearance of nausea as occurs during the tryptophan depletion test.
Several factors complicate interpretation of our data. First, the metergoline levels in our patients were higher than those in our comparison subjects. This increase was consequent to fluoxetine, which appears to decrease the metabolism of metergoline. Hence, it is possible that the increased symptoms seen in the patients represent exposure to higher levels of metergoline. This possibility is mitigated by three observations. First, symptoms either appeared or were most significant 24 hours after the procedure when metergoline levels were undetectable, diminishing the likelihood that the effects represented acute toxicity consequent to elevated levels. Second, the antidepressant efficacy of estradiol was similarly reversed by metergoline in a group of perimenopausal women with metergoline levels similar to those obtained in the comparison subjects in this study
(28). Third, no differences were observed between patients and comparison subjects in the ability of metergoline to stimulate prolactin or decrease body temperature. Hence, two physiological measures of the effects of metergoline showed comparable response at the end of the metergoline infusion.
A second complicating factor is the observation that norfluoxetine levels significantly decreased 4 hours after metergoline administration. While this decrease in the absence of an increase in fluoxetine levels is, frankly, difficult to explain, we do not believe this confounds interpretation of our data for three reasons. First, at the time of symptom appearance (24 hours), norfluoxetine levels were, if anything, nonsignificantly higher with metergoline compared with placebo. Second, symptoms at 24 hours were significantly correlated with metergoline levels but not with levels of either fluoxetine or norfluoxetine at 4 hours. Finally, the precipitation of symptoms by a clearly transient decrease in norfluoxetine (but not fluoxetine) levels would be at variance with the usual minimal consequence of a missed dose of medication. Nonetheless, we cannot rule out the possibility that a decrease in serotonin function consequent to a transient decrease in norfluoxetine levels resulted in the affective relapse seen 24 hours later.
The results of this study support the view that fluoxetine’s efficacy in premenstrual dysphoric disorder may be mediated by altered serotonergic neurotransmission. Further studies that use either receptor subtype-specific agonists or antagonists or, ideally, radioligands to image the changes in receptor systems with SSRI treatment will help further elucidate the mechanisms involved in the therapeutic efficacy of these agents in premenstrual dysphoric disorder. Such investigative techniques may also help determine whether abnormal serotonergic function underlies both the pathophysiology of premenstrual dysphoric disorder and the unique treatment response characteristics of women with this disorder.