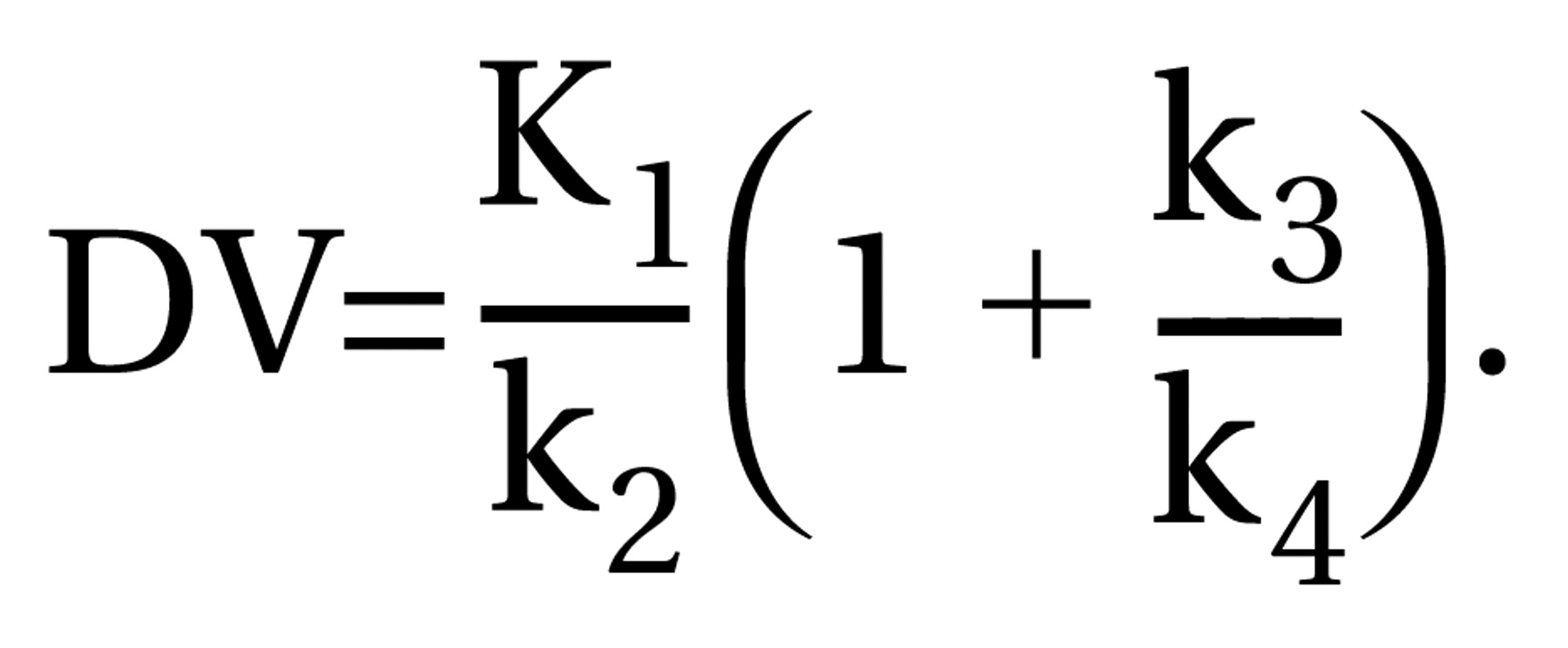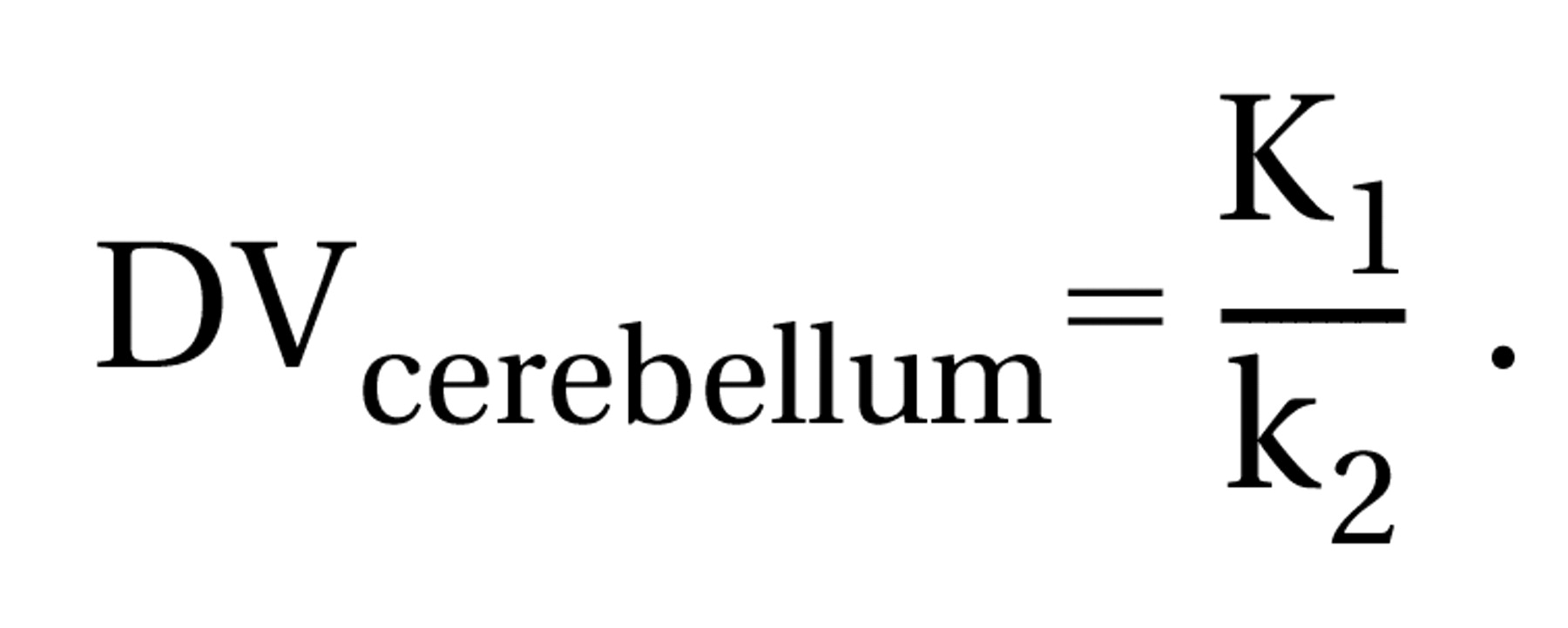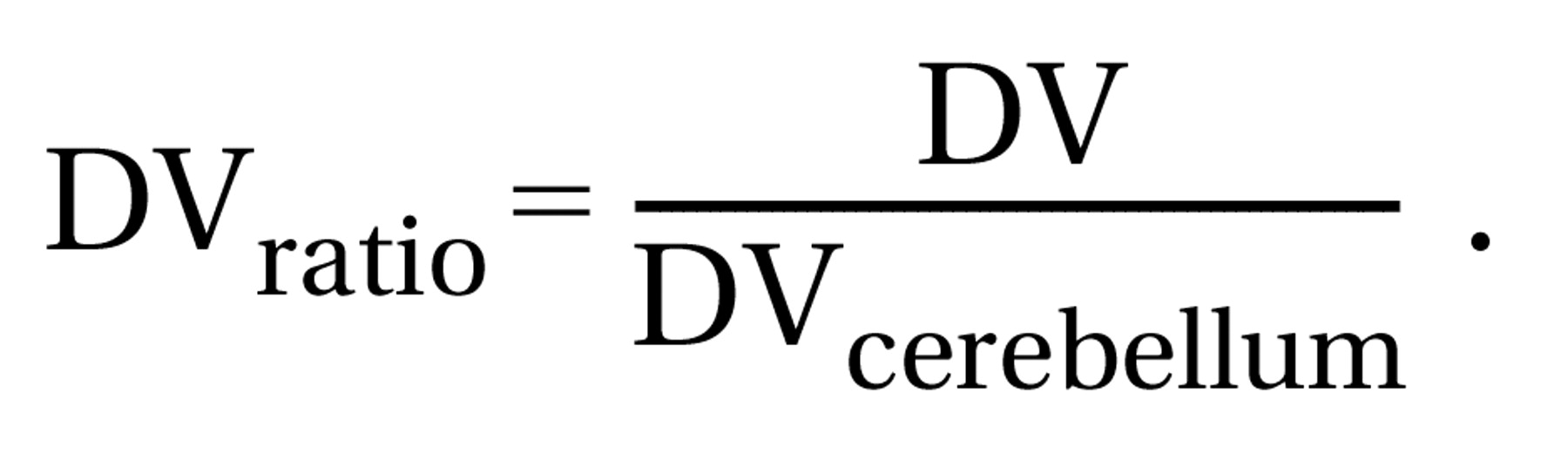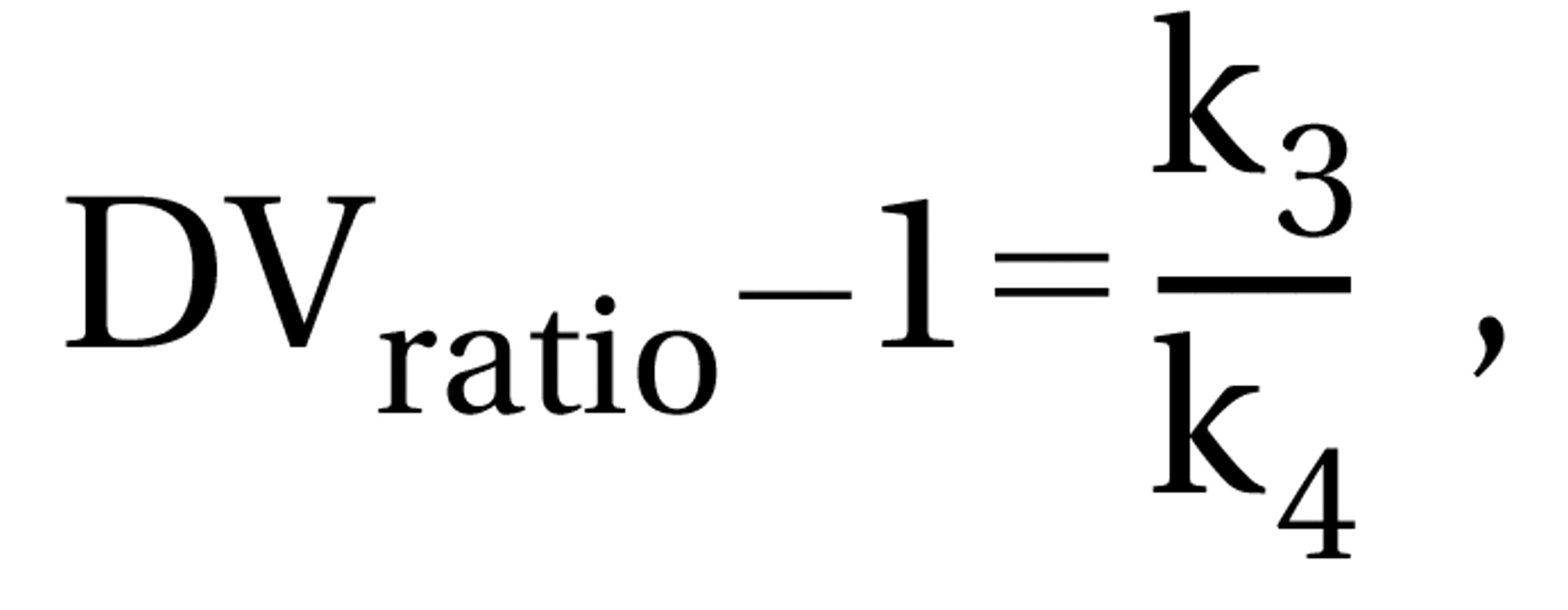The serotonin (5-HT) neurotransmitter system is widely distributed in the central nervous system and is involved in modulating mood, appetite, sleep, sexual activity, aggression, and psychomotor activation. Studies of 5-HT
2 binding density have shown age-related changes in rats
(1) and in humans studied through postmortem examination
(2,
3) and in vivo imaging studies
(4–
10). Studies using autoradiography have examined the effect of age on 5-HT
2 receptors in human brain tissue postmortem, showing a marked decrease with age. All have shown decreased receptor levels, but they have pointed to different age relationships. Marcusson et al.
(11) found the loss of 5-HT
2 receptors to occur primarily after age 60, with an approximate 50% overall decrease by the 10th decade. In contrast, Gross-Isseroff et al.
(3), using ketanserin binding, found a decrease in 5-HT
2 receptors in prefrontal cortical homogenates that leveled off in later life.
Several in vivo studies originally identified a loss of 5-HT
2A receptors with age
(4–
6). However, these studies were conducted with
11C-labeled 3-
N-methylspiperone, a neuroleptic ligand that binds preferentially to both dopamine D
2 and 5-HT receptors. Although the ratio of serotonin to dopamine receptors is high in the prefrontal cortex, and thus spiperone binding is thought to measure largely 5-HT receptors, the influence of D
2 receptors on 5-HT
2 binding in the prefrontal cortex is uncertain. Since these studies did not quantify D
2 receptors separately from 5-HT receptors, it was difficult to know the specific relationship of age to 5-HT receptors. This led to later work that examined the effect of age on 5-HT
2A binding with more selective 5-HT
2 ligands. These studies using more selective ligands
(7–
10,
12) also showed age to be a significant covariate in imaging studies of 5-HT
2A receptors. One study
(8), however, examined only a single large prefrontal cortical region, another
(9) examined a limited age range, with a mean age of only 32 years, and one
(10) compared a younger group (mean age=23 years) to an elderly group (mean age=69 years) without the full age spectrum. Using a different ligand, 2-[
123I]ketanserin, and single photon emission computed tomography, D’haenen et al.
(12) also found age-related decreases in 5-HT
2 uptake. In the current study we used [
18F]altanserin to investigate the relationship between 5-HT
2A receptor binding and age across a broad age range in a set of widely distributed brain regions. In addition, in some regions we made regional brain volumetric measurements in order to examine concomitant loss of brain volume.
Method
Subjects
This study was approved by the Washington University Human Subjects Committee. Twenty-two normal comparison subjects aged 21 to 69 years were recruited from the surrounding community. All were right-handed and screened to exclude acute physical illness or any acute or prior psychiatric disorder by review of systems, physical examination, medical records, laboratory testing, and structured interview. Written informed consent was obtained from all subjects. In addition, the Hamilton Depression Rating Scale was administered to exclude any subject with a current score of 8 or higher.
Imaging
A previously reported method
(13) for synthesis of [
18F]altanserin to image the 5-HT
2A receptors was used. Synthesis of [
18F]altanserin yielded batches up to 75 mCi with high radiochemical purity (>99%) and specific activity greater than 2000 mCi/mmol. Positron emission tomography (PET) images were obtained by using the Siemens 961HR PET scanner (Siemens/CTI, Knoxville, Tenn.). Head position was stabilized by use of a softened thermoplastic mold. The subject was placed such that the lowest imaging plane was approximately at the same level as and parallel to the canthomeatal line. A 10-minute transmission scan using rotating rods of
68Ge/
68Ga was used.
PET imaging of 5-HT2A receptors was begun by using a 20-mCi intravenous injection of [18F]altanserin and initiating a 90-minute dynamic PET image collection (12 6-second images, eight 60-second images, and 16 300-second images). In addition, rapid hand-drawn 0.6-ml arterial blood samples were obtained and centrifuged to enable counting of plasma activity. Five additional 3-ml blood samples were obtained to allow determination of labeled metabolites. Metabolite corrections of the blood samples were performed by using thin-layer chromatography with methanol as the liquid phase.
Magnetic resonance imaging (MRI) studies were performed by using a standard protocol on a 1.5-T Sonata system (Siemens Medical Systems, Erlanger, Germany), as described elsewhere
(14). Regions of interest were determined for the hippocampus, posterior medial prefrontal cortex, bilateral anterior medial gyrus rectus, anterior cingulate, occipital cortex, and cerebellum. The hippocampus was defined as described earlier
(14). The posterior medial prefrontal cortex was defined on coronal slices ventrally as the cortex of the medial aspect of the gyrus rectus (also architectonic area 14)
(15) that is continuous with the subgenual prefrontal cortex (also architectonic areas 24, 25, and 32m)
(15). This region was defined as beginning anteriorly at the genu of the corpus callosum and extending to the third ventricle. The bilateral anterior medial gyrus rectus was defined on coronal slices; the left and right cortex of the medial aspect of the gyrus rectus began anteriorly at the frontal pole and extended to the genu of the corpus callosum. The anterior cingulate was defined as a representative portion of total cingulate cortex gray matter bounded superiorly by the roof of the lateral ventricles, inferiorly by the orbital cortex, posteriorly by the genu of the corpus callosum, and anteriorly by the posterior branch of the cingulate gyrus. For the occipital cortex, a 2×2×2-cm cube was positioned over the primary visual cortex at least 8 mm away from the cerebellum. The cerebellum was defined by a 4×4-cm-wide rectangle centered in the middle of the cerebellum and at least 8 mm from any cortical structure. Stereological techniques
(14,
16) were used to determine volumes for the hippocampus, posterior medial prefrontal cortex, and medial gyrus rectus, which are anatomically discrete brain structures. Finally, a region of interest encompassing the whole brain (or global brain) was derived from all of the pixels on the MRI within the brain but excluding the cerebellum and brainstem.
PET image frames were coregistered to each other and then coregistered to the MR data set by using in-house software
(17; Snyder, unpublished data). Data on the regions of interest were then extracted from the realigned high-resolution PET images.
Modeling
Values for the plasma metabolites were expressed as percentages of unchanged altanserin for each time, and the data were fitted to an exponential function. The resulting function was used to correct the plasma activity data to yield the unchanged [18F]altanserin activity curve.
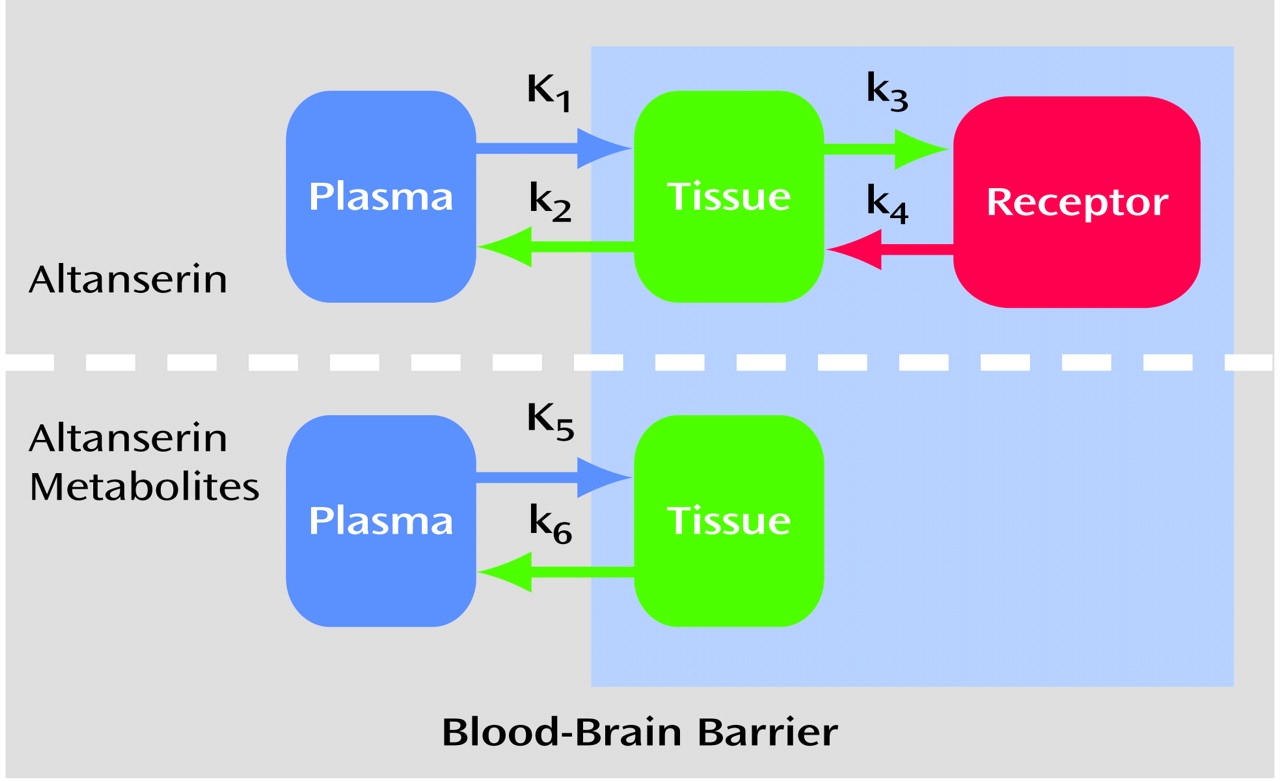
A four-compartment kinetic analysis model was used to account for transport of the [
18F]altanserin tracer into the brain, nonspecific binding to the brain tissue, and specific binding to the 5-HT
2A receptor. The labeled metabolites of altanserin in the brain were modeled as well
(23) (
Figure 1) on the basis of evidence that one or more of the metabolites of [
18F]altanserin can cross the blood-brain barrier. These metabolites show no binding to the 5-HT
2A receptor
(19–
21).
While there is a small but finite number of 5-HT2A receptors in the cerebellum
(22), the contribution from receptor binding is assumed to be negligible, and the cerebellum can be used to estimate the nonspecific binding. The cerebellum data were processed first, yielding estimates of the plasma volume, bidirectional transport rate constants, and nonspecific binding of [
18F]altanserin and the metabolites
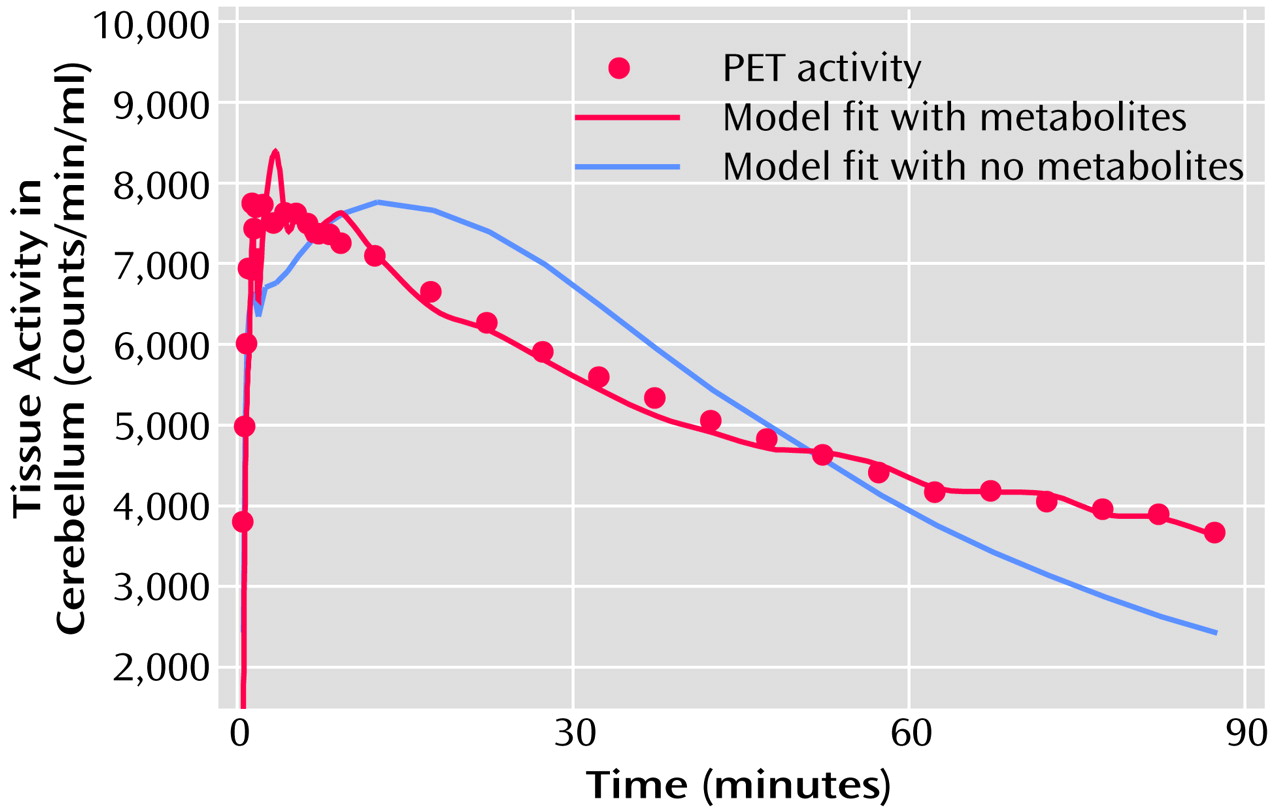
(18). Thus, the cerebellum was modeled by using two single compartments simultaneously (labeled altanserin and labeled metabolites). This model fits the cerebellum very well (
Figure 2). No receptor-bound component was needed in the cerebellum for adequate model fitting when metabolites were considered.
Next, assuming that the nonspecific binding of altanserin and the altanserin metabolites are relatively constant throughout the brain
(13,
22), we held the ratios K
1/k
2 and K
5/k
6 fixed and analyzed the other individual regions, which resulted in estimates of K
1, k
3, and k
4. The [
18F]altanserin distribution volume (DV) is calculated by Equation 1:
The cerebellum distribution volume is used to correct for variations in nonspecific binding in plasma and is simply calculated as in Equation 2:
To express the number of receptors in the brain tissue, one uses DVratio–1, in which DVratio is calculated by Equation 3:
Note that with algebraic substitution of Equations 1 and 2 into Equation 3, DVratio–1 can also be calculated as in Equation 4:
in which k
3 and k
4 are derived from the model. Furthermore, the k
3/k
4 ratio is proportional to binding potential
(24).
Statistical Analysis
Pearson correlations were calculated to determine the relationship between age and estimates of receptor binding, DV
ratio–1. Linear and quadratic relationships were fitted to the data. Visual inspection of the data suggested that a linear regression does not entirely explain the behavior of receptor binding changes over age. To explore this behavior we tested a quadratic relationship of age and receptor binding against a linear relationship. The statistical significance of the quadratic term was determined by t test. To further illustrate this potential nonlinear effect we divided the data at the midpoint of the age range (age 45) and plotted the two age groups separately. Then, using a method to compare slopes (see reference
25, pp. 292–295), we tested whether the slopes of receptor loss in the younger subjects were different from the slopes of the older subjects. The method for testing hypotheses about the equality of two population regression coefficients (slopes) involves the use of Student’s t test in a manner analogous to that for testing differences between two population means. It should be noted that these methods were used merely to characterize these apparent deviations from linearity but not to determine the exact relationship between age and receptor binding, which would require additional data and assumptions regarding the biological processes involved. In addition, the correlations between the brain structure volumes and age were determined by using Pearson coefficients.
Results
The subjects consisted of 16 women and six men. They ranged in age from 21 to 69 years (mean=43.4, SD=13.3). Among women only, the range was 25–69 (mean=46.3, SD=13.7), and among men the range was 21–44 (mean=35.7, SD=8.9). Values of DV
ratio–1 are displayed in
Table 1. The distribution volume for the cerebellum was not correlated with age (r=0.33, df=21, p=0.14). The mean decrease in 5-HT
2A binding per decade of age is shown in
Table 1. For each brain region the coefficient for the correlation between binding potential and age is also displayed.
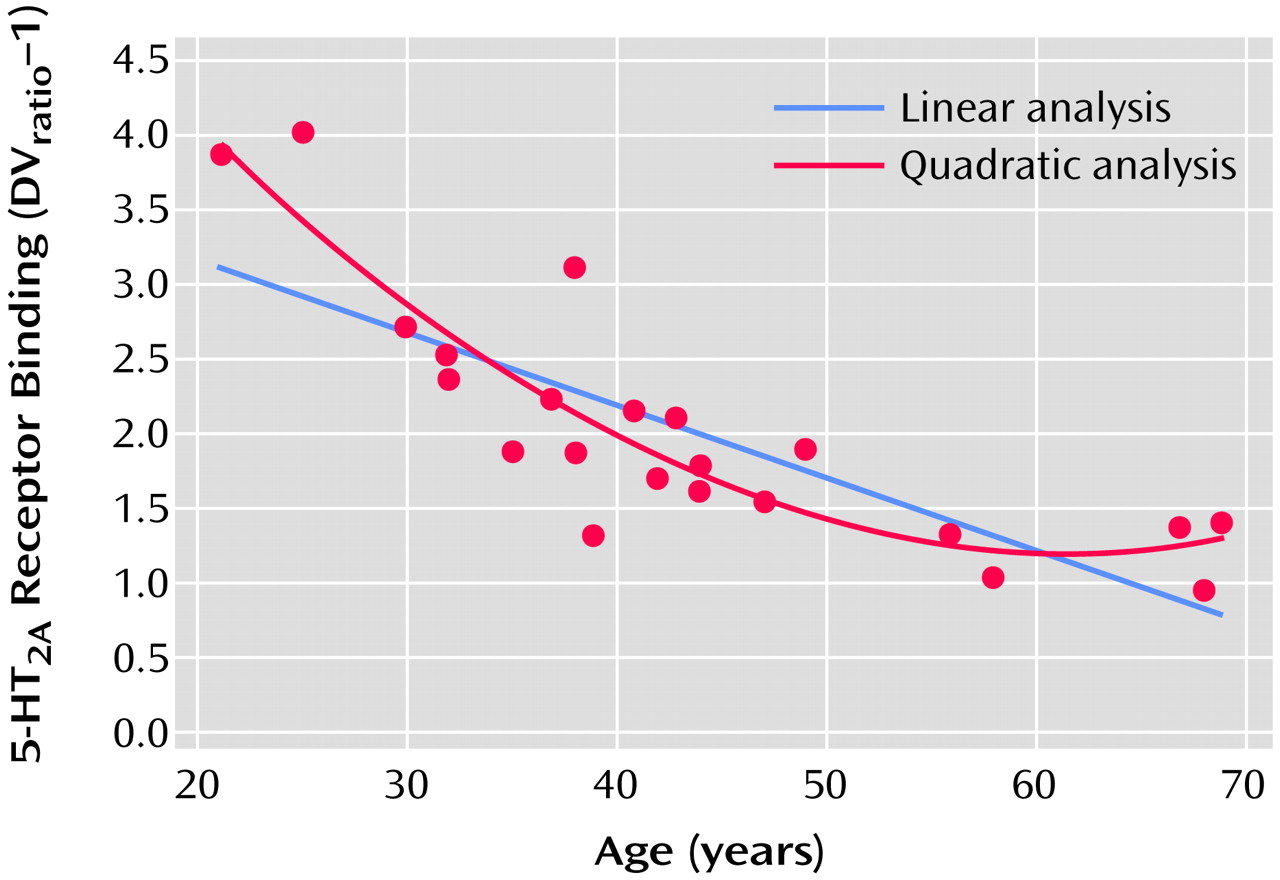
The proportion of variance accounted for (R
2) was calculated for linear and quadratic fits of the 5-HT
2A binding data with age (
Figure 3). The relationship of receptor binding to age was significant with both the linear fit (r=0.80, df=21, p<0.0001) and the quadratic fit (r=0.89, df=21, p<0.0001) of the data. The addition of the quadratic term added significant information over the linear fit for the global data (linear R
2=0.64, quadratic R
2=0.79), occipital cortex (linear R
2=0.59, quadratic R
2=0.71), gyrus rectus (linear R
2=0.59, quadratic R
2=0.67), and posterior medial prefrontal cortex (linear R
2=0.67, quadratic R
2=0.85) but not for the anterior cingulate or hippocampus. Visually, this appears as a rapid fall in receptors during early adulthood and midlife followed by a leveling off of the decrease in 5-HT
2A receptor binding with age (
Figure 3). To test whether the additional quadratic term is significantly different from zero (i.e., the quadratic fit adds significant information over the linear fit) we performed a t test (see reference
25, p. 365). The quadratic term was significantly different from zero for the global data (t=3.83, df=19, p<0.001), occipital cortex (t=2.91, df=19, p<0.009), gyrus rectus (t=2.16, df=19, p<0.04), and posterior medial prefrontal cortex (t=4.68, df=19, p<0.001) but not the anterior cingulate (t=1.75, df=19, p=0.10) or hippocampus (t=1.16, df=19, p=0.26).
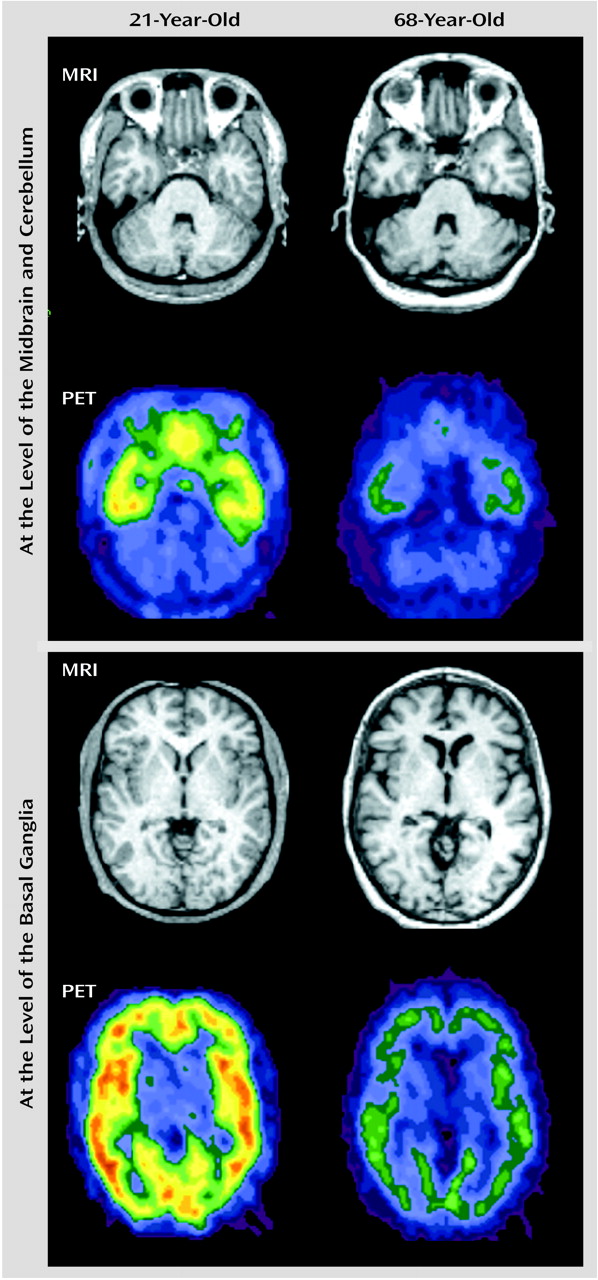
Figure 4 displays representative PET slices and the coregistered MRI scans for a 21-year-old subject and the same slices for a 68-year-old subject.
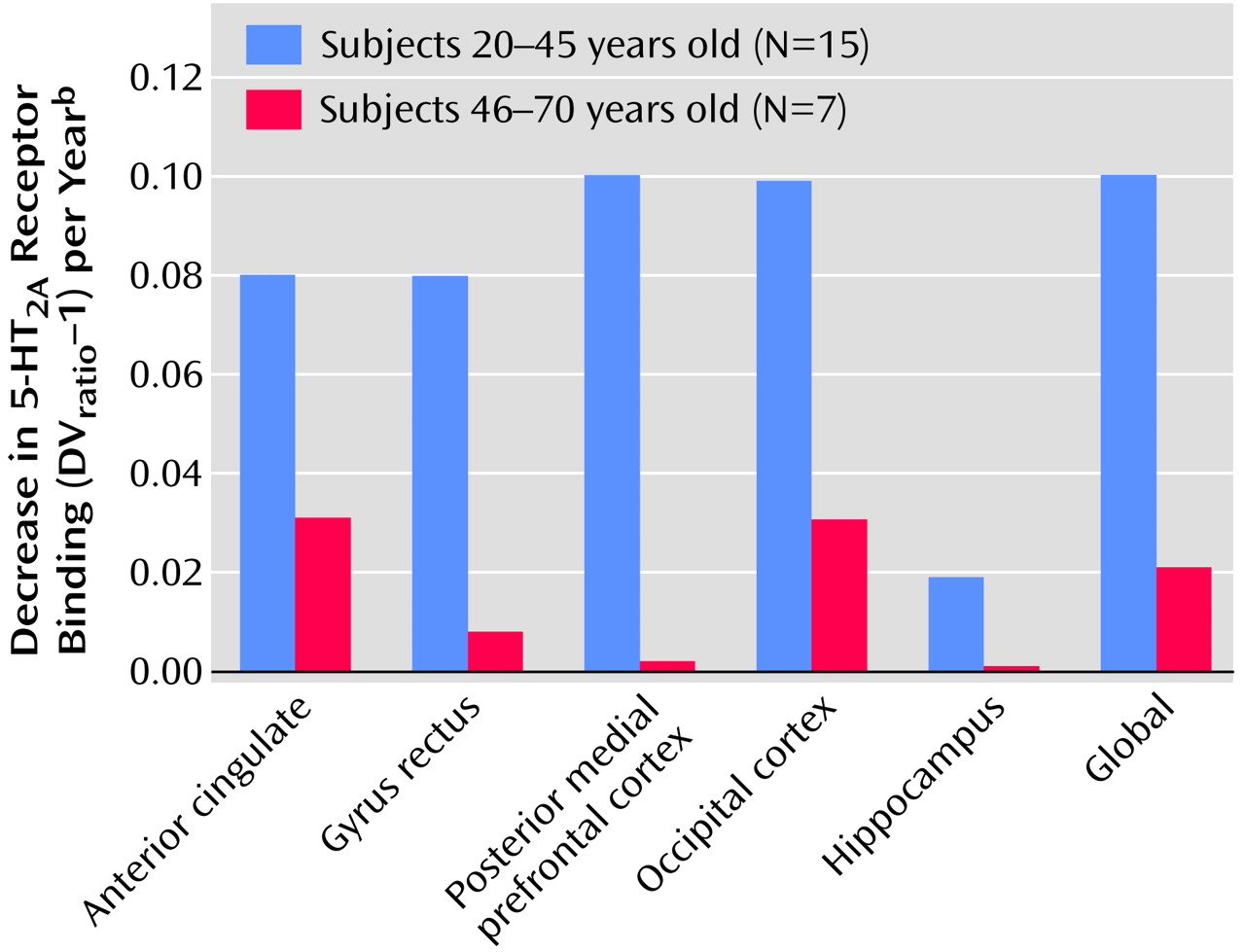
To illustrate the potential change in slopes over the life span more directly, the age-related decline in receptors was examined separately for young and old subjects. As shown in
Figure 5, the decline in receptors was much greater for younger (age=20–45 years) than for older (age=46–70 years) subjects in the anterior cingulate (t=4.35, df=18, p<0.0001), gyrus rectus (t=4.33, df=18, p<0.0001), posterior medial prefrontal cortex (t=4.22, df=18, p<0.001), occipital cortex (t=3.65, df=18, p<0.002), hippocampus (t=4.31, df=18, p<0.001), and global brain (t=3.69, df=18, p<0.002).
To compare age-related receptor loss to age-related volume loss, the average percentage loss in volume per decade was calculated for each region. These losses were 8.9% for the gyrus rectus, 4.6% for the posterior medial prefrontal cortex, and 3.4% for the hippocampus. Some regions for which receptor data are given do not have corresponding volumetric data, since some regions (cerebellum, occipital cortex, and anterior cingulate cortex) were measured as cubic subvolumes rather than by meaningful anatomical boundaries (see Method section). PET methods do not currently allow complete correction of partial volume effect without assumptions regarding activity in the tissues surrounding the region of interest. Merely knowing the volume of a structure without detailed knowledge of the activity of surrounding tissue makes it difficult to determine partial volumes, although there have been various attempts at estimation
(9,
26).
Discussion
In this study we found dramatic decreases in 5-HT2A receptors in normal healthy subjects across a broad range of ages and in widely distributed regions. The receptor loss was large—70% from the levels at age 20, progressive through the fifth decade and then leveling off. Our results extend previous findings that age was a significant covariate in imaging studies of 5-HT2A receptors. Our study fully modeled ligand behavior with full quantitative receptor kinetics, including metabolites, by using blood sampling. Unlike some investigators, we did not simplify receptor kinetics to tissue ratios or a Logan plot. The ligand we used, [18F]altanserin, binds specifically to the 5-HT2A receptor. We examined the relationship between 5-HT2A receptor binding in a variety of cortical and limbic regions. We looked across an age span of 21–69 years, although we had only two subjects under the age of 30 and few elderly subjects.
Like others
(4–
7,
9,
10,
12), we found receptor loss in widely scattered brain regions. In the posterior medial prefrontal cortex (areas 24, 25, and 32m), gray matter of the gyrus rectus (area 14), anterior cingulate, hippocampus, and occipital cortex the relationships between age and receptor density were similar. When a linear equation was used to fit the data, there was a slope of approximately 16%–18% per decade in these regions. Further analysis suggested that for most regions studied the loss of receptors is not a purely linear function. It appears to be greatest in midlife and to level off after age 50 years. A parabolic relationship with aging was first reported by Gross-Isseroff et al.
(3), who used [
3H]ketanserin specific binding to postmortem prefrontal cortex determined by autoradiography. They reported a progressive decrease in 5-HT
2 receptors until age 60, followed by flattening out of the curve with no further loss in receptors (and actually a slight upswing after age 60). In comparing the fit of our data from a linear regression equation to the fit from a quadratic equation, the R
2 value increased substantially for receptor binding in the whole brain and in the posterior medial prefrontal cortex, gyrus rectus, and occipital cortex but not in the hippocampus or anterior cingulate cortex. This suggests that in the former regions a nonlinear falloff of receptors is a better approximation, whereas in the latter regions the relationship appears to be more linear. It is unclear why the loss of receptors would occur more in midlife than late life.
The large changes in 5-HT
2A receptor density were not associated with similar changes in brain volume. The literature on change in brain volume with age contains varied results based on a wide variety of study groups. Some studies have shown decreases in overall brain volume with age
(27) or age-related atrophy in particular regions
(28). In general, studies that have screened most carefully to exclude subjects with medical problems have shown the smallest decreases in brain volume
(29).
We cannot examine quantitatively the relationship between receptor density and brain volumes in particular brain regions. The PET measure of radioactivity in the volume of interest receives an unknown contribution from all surrounding tissues because of the finite resolution of the scanner. However, it appears that the tissues we examined had a modest change in volume with age, much smaller than the corresponding receptor loss from age 20 to 70. While our data do not preclude neuronal dropout as an explanation for the decrease in 5-HT2A binding, they do imply that a general neuronal loss does not exist to a degree sufficient to explain the large decreases in 5-HT2A binding. Therefore, if the falloff in 5-HT2A receptor binding is due to neuronal loss, it would have to be a loss relatively specific to 5-HT2A cells.
The meaning of this age-related loss in 5-HT
2A receptors is not clear. There is some evidence for decreased serotonergic function with age. In a comparison of prolactin responses to
d,l-fenfluramine (a serotonin-releasing agent) in depressed and comparison subjects, Lerer et al.
(30) found that the comparison subjects exhibited a significant age-by-challenge-by-time interaction. Peak minus baseline prolactin responses were negatively correlated with age in both women and men. In a group of subjects with major depression, Degl’Innocenti et al.
(31) showed a correlation between prolactin response to
d-fenfluramine and performance on four of five selected Wisconsin Card Sorting Test variables, which was also negatively correlated with age. These results further support a relationship between decreases in serotonergic activity and age. Since there appear to be losses in several neurotransmitter systems, including cholinergic systems as well as serotonergic and dopaminergic systems
(32,
33), it is possible that functional deficits that occur in old age result from a combination of deficits in these systems. There also may be a large redundancy in these systems, such that loss of even a large number of receptors may not cause a detectable loss of function.