Prompt Identification of Alzheimer’s Disease With Brain PET Imaging of a Woman With Multiple Previous Diagnoses of Other Neuropsychiatric Conditions
Case Presentation
Ms. A was a 53-year-old woman who came to the memory clinic with the chief complaint that it was “hard to put thoughts together.” She reported a progressive decline in her memory abilities over the previous 4 years, for which she had undergone general medical, neurological, psychiatric, and formal neuropsychological evaluations. She had been variously diagnosed as having depression, migraines, fibromyalgia, attention deficit disorder, and posttraumatic stress disorder stemming from childhood abuse. Her medications included the selective serotonin reuptake inhibitor citalopram (40 mg/day for the previous 18 months), levothyroxine for hypothyroidism secondary to Hashimoto’s thyroiditis, and estrogen-replacement therapy.
Past History
A review of Ms. A’s records from an outside health organization revealed that her internist first noted a cognitive problem 2 years earlier when he recorded “memory loss” of 1 years’ duration. At that time he also diagnosed depression, obtained laboratory screening test results, referred Ms. A to a neurologist, and scheduled her for a return visit in 6 weeks.The next month, Ms. A, accompanied by her husband, had her first visit with the neurologist. He noted that a computerized tomographic scan of the brain and tests of her CSF, obtained in the remote past for workup of headache, were unremarkable. Her examination was normal, except for her mental status: “She appeared anxious and hyperventilated at times.” Her neurologist concluded, “Most of the patient’s symptoms are likely psychiatrically based. She is currently in therapy and being treated. Nevertheless, because of the indication of deteriorated function at work, documented by her husband [with whom she ran a small restaurant], I would like to get [magnetic resonance imaging] MRI of the brain to rule out any frontal lobe disease.”An MRI of her brain performed without contrast the next week showed a normal cortex, brainstem, basal ganglia, thalami, and ventricles. A “hazy 1-cm area of abnormal signal in the right parietal deep white matter” without mass effect was observed on fluid-attenuated inversion recovery and T2-weighted images but not on T1-weighted images. A postcontrast MRI, performed 2 weeks later, demonstrated a nonenhancing “vague area of slightly increased signal in the area of the previously noted increased signal” on one axial image. The radiologist concluded, “The finding may represent a small area of ischemic change.”Two and one-half months after the second MRI, Ms. A returned to her internist, who noted, “Memory lapses persist.” His plan was to follow up with the neurologist, refer Ms. A to the behavioral health unit, and have her return in 6 weeks. When she returned 6 weeks later, the internist again noted that “Memory lapses persist,” and his assessment was “depression” and “memory loss.”Two weeks later, Ms. A had her second visit with the neurologist. He noted “a small area of T2 change that is unchanged on serial MRI, consistent with her history of migraine.” His impression stated, “The differential does include the possibility of frontal lobe dementia, but the patient has no change in personality or other frontal lobe symptoms, and she also has lack of atrophy on brain MRI. We still cannot rule this out and will need to observe her over time.”After another 6 months (1 year after Ms. A’s first visit to the internist for memory loss), she returned to her internist, who again noted, “Memory lapses persist.” His assessment indicated, “1) Hypothyroidism, 2) depression, 3) fibromyalgia.”Eight and one-half months later, Ms. A’s husband brought her to a licensed psychologist for formal neuropsychological testing, again expressing the concern that his wife was “experiencing cognitive problems.” Ms. A told the psychologist that she was afraid she was losing her mental capacity: “I forget things, I forget people I meet, lose things in my mind.” The psychologist reported that Ms. A’s verbal IQ was in the normal range, but her performance IQ was markedly deficient. The psychologist’s summary stated, “The results suggest neurological difficulties. It can be said that posttraumatic stress disorder factors into the results.…However, neurological problems should be ruled out ASAP,” and he further recommended individual counseling, as well as marriage counseling, because of “her concern for her husband’s feelings about her difficulties.”
Evaluation at the Memory Clinic
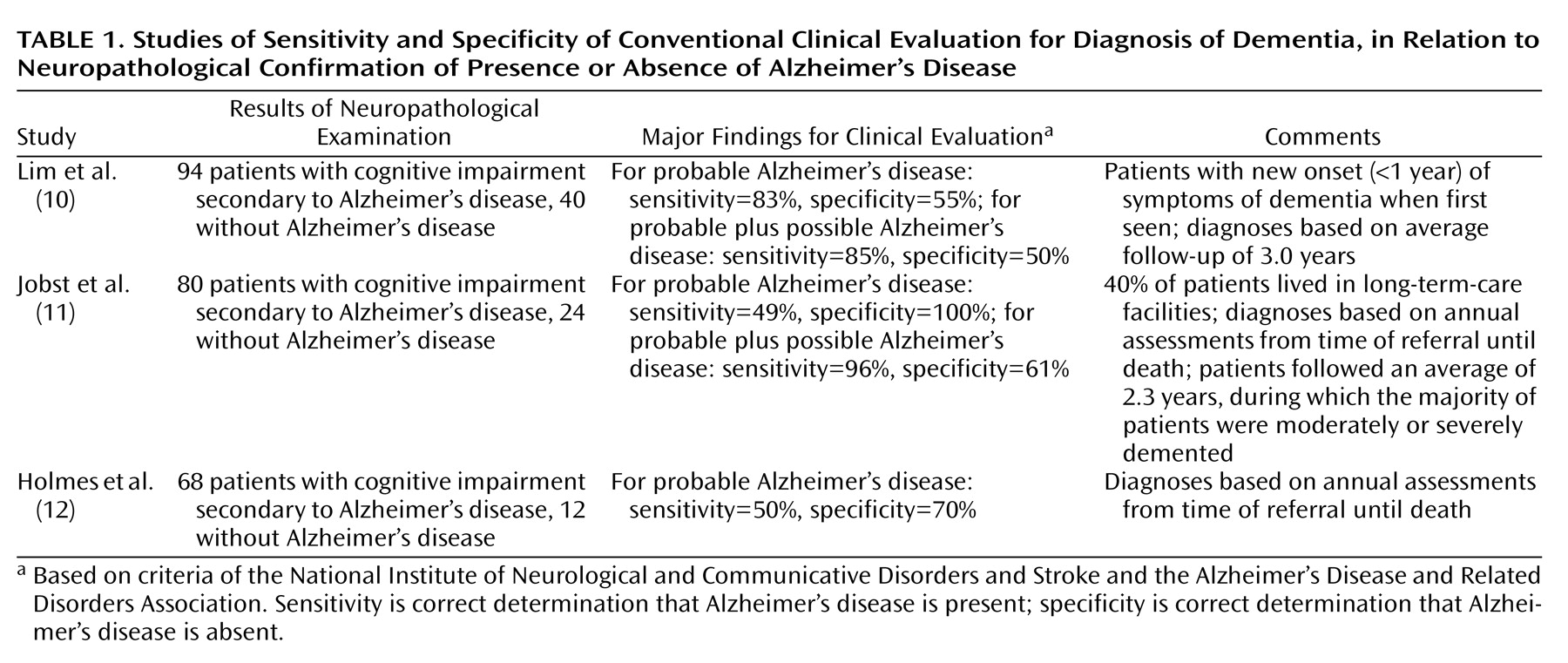
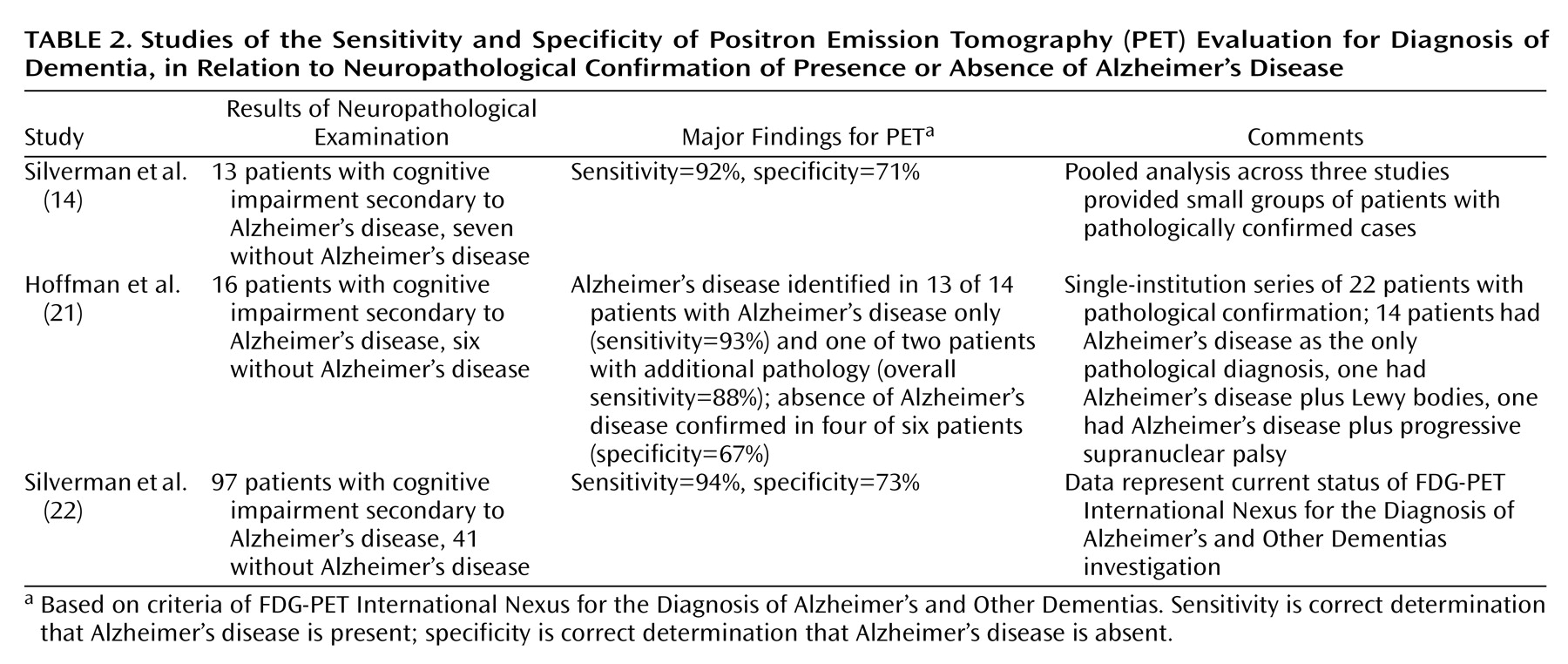
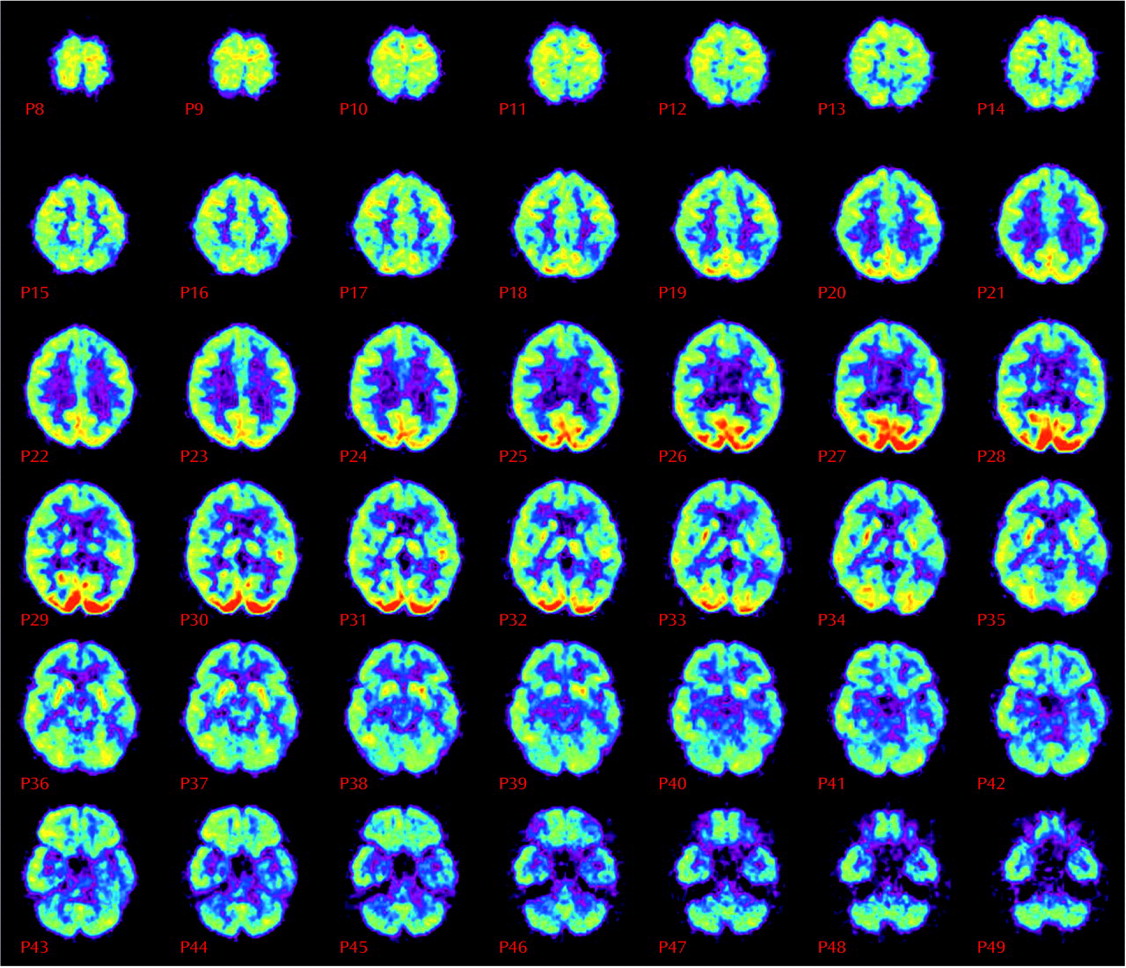
After another 9 months, still without a specific diagnosis for her memory complaints, Ms. A was evaluated for the first time by a geriatric psychiatrist at the memory clinic. At her psychiatric examination, Ms. A was reported to be “alert, mildly anxious, euthymic, friendly, cooperative, with bright affect.” Her performance on the Mini Mental State Examination (MMSE), however, was clearly abnormal; she scored only 18 of a possible 30 points and remembered none of three possible items for short-term recall. Once her previous brain imaging studies had been reviewed, a PET scan, which images regional brain metabolism with the use of [18F]fluorodeoxyglucose (FDG), was obtained. The scan revealed diffuse and moderately severe cortical hypometabolism, especially affecting the bilateral midparietal cortex and left inferior frontal and temporal cortex, but sparing the bilateral sensorimotor and visual cortices (Figure 1). The nuclear medicine physician who interpreted the results noted that this pattern is characteristic of neurodegenerative dementia—most commonly, Alzheimer’s disease. The PET scan was followed by an MRI study with T1-weighted, T2-weighted, and fluid-attenuated inversion recovery sequences, which was normal, with no evidence of infarction or white matter lesions. A week after the PET, a neuropsychologist administered a test battery to Ms. A and concluded that although “other diagnoses that she has received in the past, such as fibromyalgia, attention deficit disorder, posttraumatic stress disorder, and depression could be a factor in her performance, it is emphasized that they would not explain the severity or scope of her current deficits.”
Treatment Plan and Subsequent Course
The psychiatrist who originally saw Ms. A diagnosed probable Alzheimer’s disease, citing the results of PET and neuropsychologic testing, as well as her unremarkable MRI. Treatment was initiated with the cholinesterase inhibitor donepezil at 5 mg/day and increased to 10 mg/day after a month. The family was also referred to the Alzheimer’s Association for education and support and was informed that Ms. A should refrain from driving. She responded well to the treatment: her MMSE score increased from 18 to 21, and she correctly remembered two of three items on the test of short-term recall. Her husband described her as “more engaging with others, more alert.” Ms. A and her husband decided to move to northern California to be closer to their children and to return to Los Angeles every 6 to 12 months for visits to the memory clinic.
Discussion
Footnote
References
Information & Authors
Information
Published In
History
Authors
Metrics & Citations
Metrics
Citations
Export Citations
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download.
For more information or tips please see 'Downloading to a citation manager' in the Help menu.
View Options
View options
PDF/EPUB
View PDF/EPUBLogin options
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Personal login Institutional Login Open Athens loginNot a subscriber?
PsychiatryOnline subscription options offer access to the DSM-5-TR® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).

