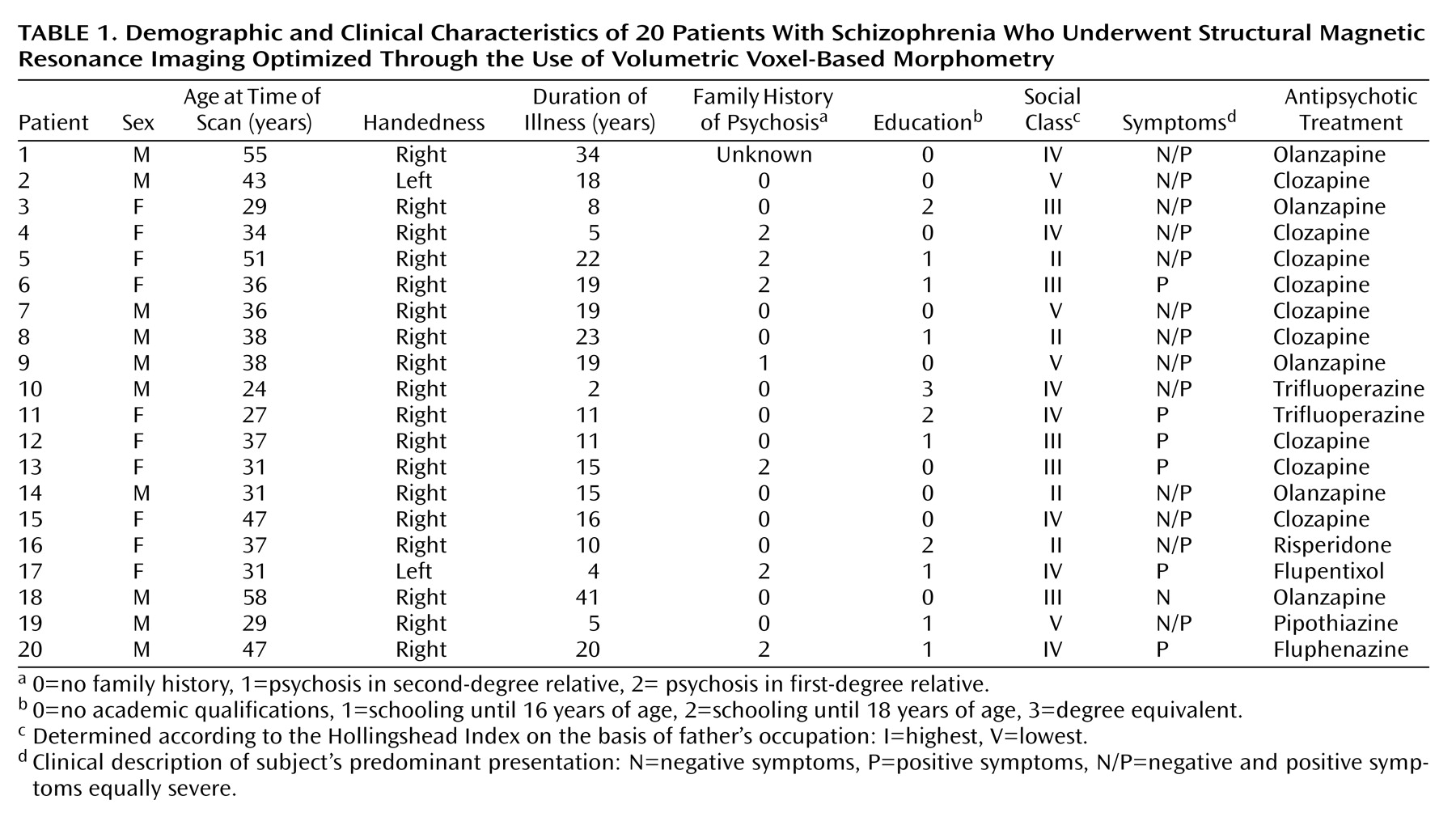
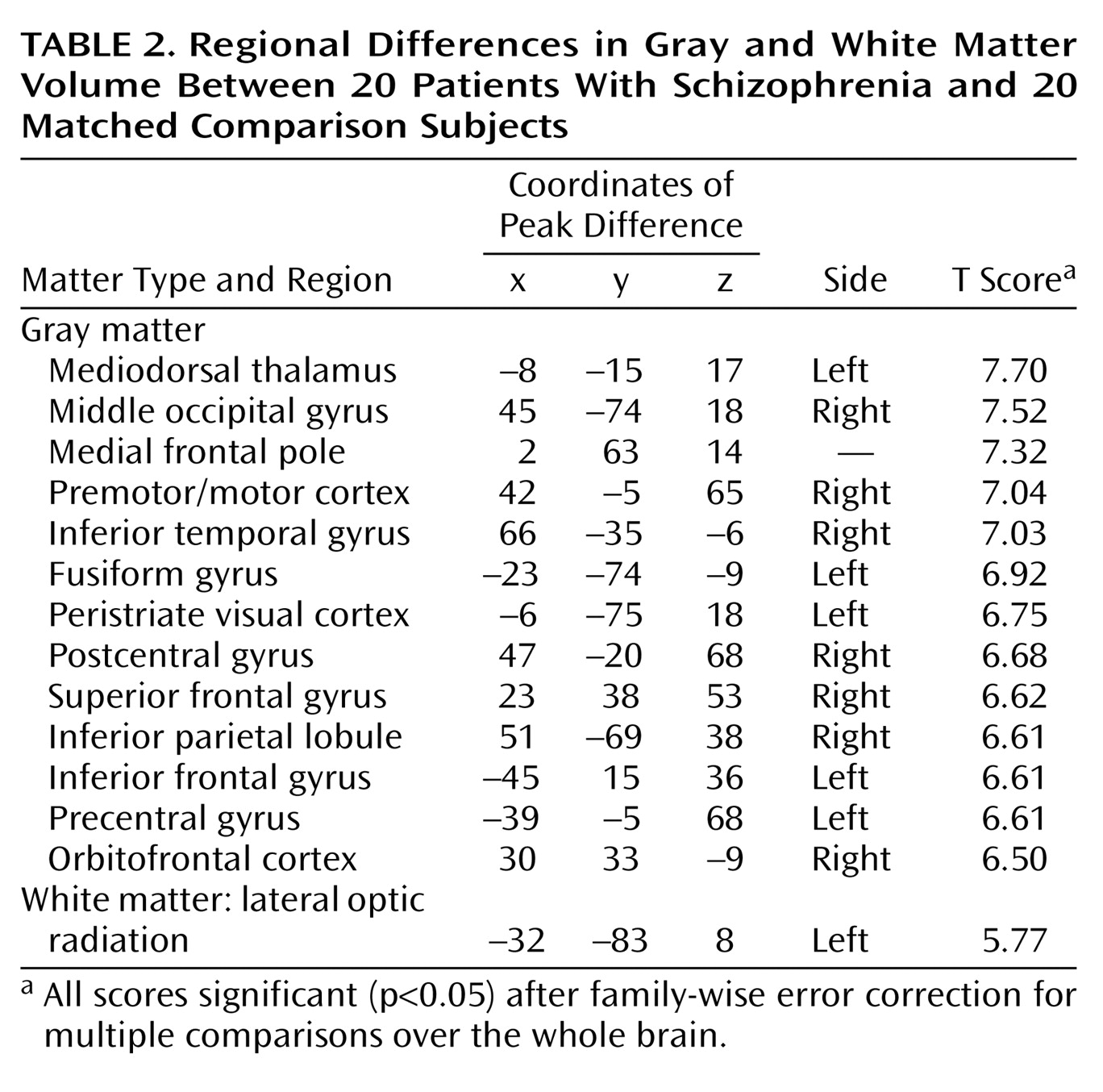
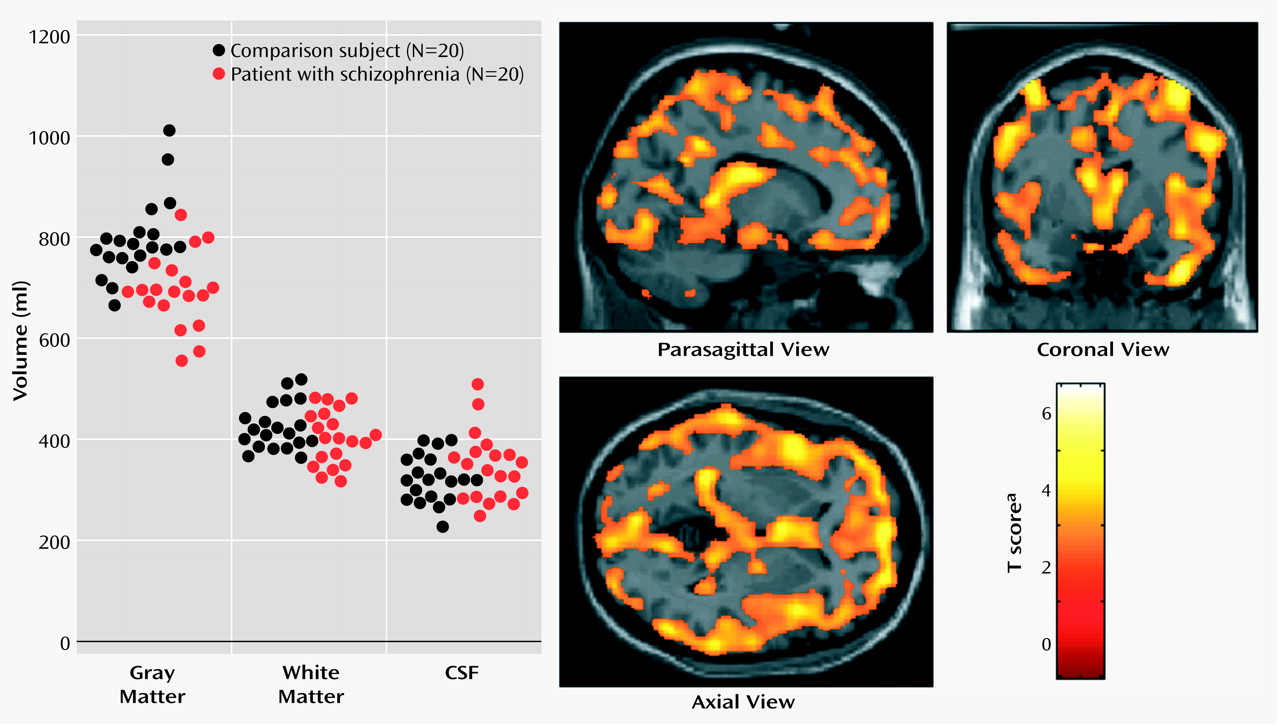
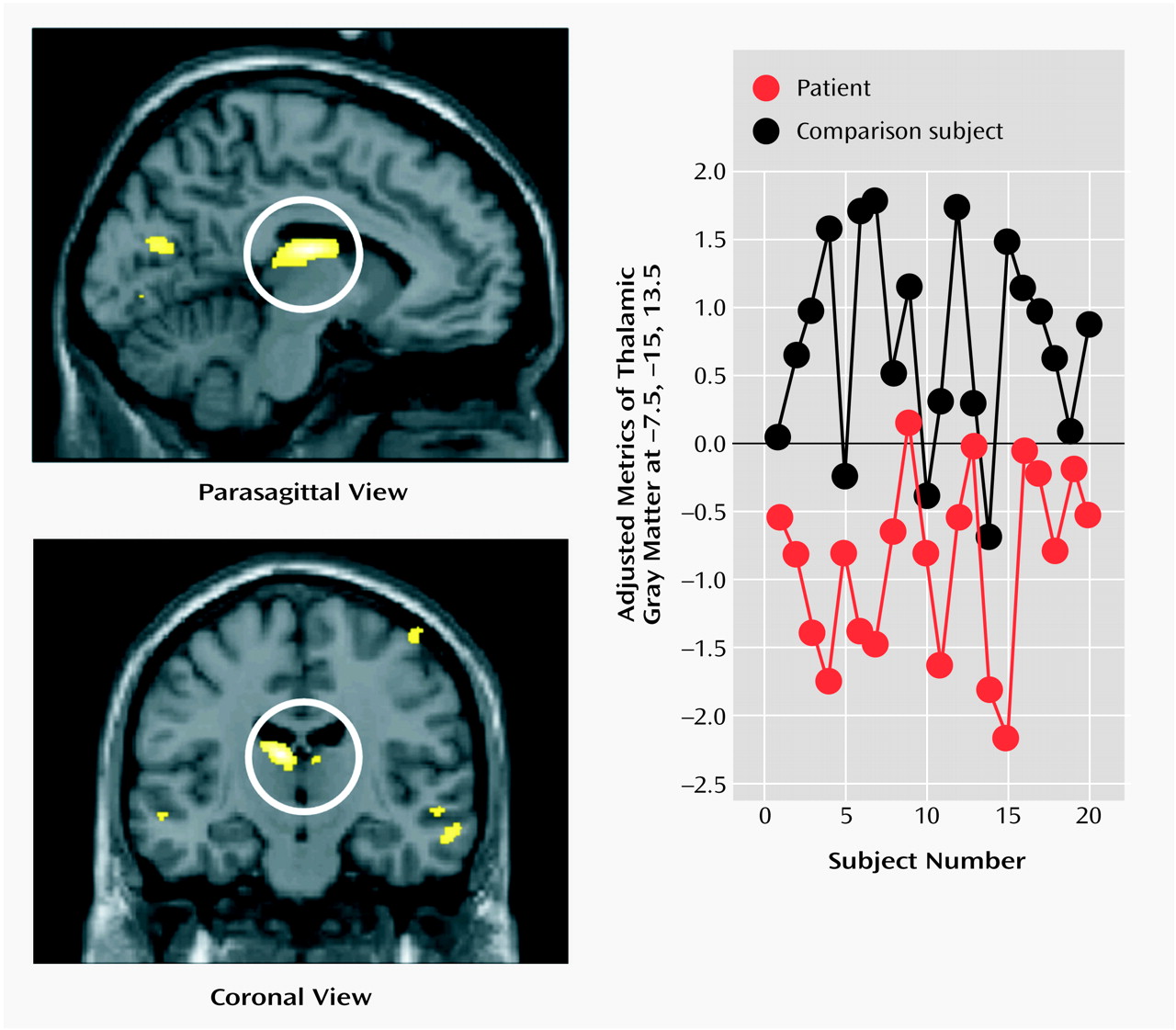
Schizophrenia is a complex mental illness characterized in acute phases by delusions, hallucinations, and disordered thinking and chronically by apathy, flat affect, and social withdrawal. The course is chronic in a high proportion of cases, although the underlying pathophysiology is unknown. Early descriptions of schizophrenia as “dementia praecox”
(1) argued for an organic degenerative basis to the disorder. However, despite a worldwide prevalence of 1% and annual service provision costs within the United Kingdom estimated at 2.6 billion pounds
(2), the neuropathological basis of schizophrenia has remained elusive. Negative cognitive symptoms characterize the neuropsychological profiles of many patients with schizophrenia. However, many formulations of the “schizophrenic mind” suggest a primary selective dysfunction of brain processes involved in filtering and gating information
(3).
Histological evidence for a neurodegenerative process underlying schizophrenia is limited by a paucity of postmortem studies and methodological inconsistencies with techniques. High-resolution in vivo brain imaging has given impetus to understanding the neurobiology of schizophrenia, including the description of changes in brain morphology that may provide insights into etiological mechanisms. Among the most consistent neuroimaging findings in schizophrenia are greater cerebroventricular size and cerebral gray matter deficits
(4–
8), which suggests the presence of diffuse brain pathology. Observations of localized morphometric abnormalities in patients with schizophrenia have been less consistent across studies. In a meta-analysis of 58 morphometric studies of schizophrenia (with the majority having employed manual tracing of regions of interest), differences in medial temporal lobe structure volumes were the most frequent observations
(8). Morphometric abnormalities in the thalamus have also been reported
(9–
11) and may reflect changes maximally localized within the mediodorsal thalamic nucleus
(12). This finding is of note, since thalamic dysfunction, potentially leading to impaired sensory filtering and gating mechanisms, has been proposed to underlie the psychological abnormalities that characterize schizophrenia
(13–
16).
Voxel-based morphometry provides an automated means of comparing structural features across scans and overcomes problems of intra- and interobserver bias and sensitivity in assessing differences in cerebral morphology between groups of subjects
(17–
19). The first application of this technique to whole-brain magnetic resonance imaging (MRI) scans of patients with schizophrenia reported gray matter deficits in the insula and prefrontal and lateral temporal cortices
(7). Similar methods have also been used to relate gray matter deficits in the medial prefrontal cortex, insula, and superior temporal cortex to severity of schizophrenic symptoms. Abnormalities in the thalamus, posterior cingulate, and basal ganglia, with ventricular enlargement, have been associated with childhood-onset schizophrenia
(20). Voxel-based morphometry has also been applied to analysis of scans obtained by using magnetic transfer imaging, an MRI technique sensitive to subtle changes in regional macromolecular integrity
(21). Frontotemporal abnormalities were noted in schizophrenic patients, and severity of negative schizophrenic symptoms reflected abnormal integrity of the medial prefrontal cortex. These and similar voxel-based morphometry studies point to a combination of diffuse gray matter volume deficits and more localized frontotemporal changes, perhaps selectively involving thalamocortical circuitry.
Recent methodological developments have optimized voxel-based morphometry techniques
(18,
19), improving sensitivity to morphometric differences and control for macroscopic features. In standard voxel-based morphometry, erroneous missegmented voxels may appear in segmented images because of systematic differences in skull size, shape, or thickness across study groups. These problems have been addressed in an optimization of the methodology by addition of further preprocessing steps to exclude nonbrain voxels before normalization and subsequent segmentation, including a fully automatic brain extraction technique, study-specific gray/white matter templates, and a modulation step that incorporates volume changes during normalization into the analysis. This modulation procedure allows assessment of specific changes in regional gray/white matter volume. In this study, these methods were applied to test the specific hypothesis that characteristic differences exist between schizophrenic subjects and healthy comparison subjects in global and regional brain morphology.
Discussion
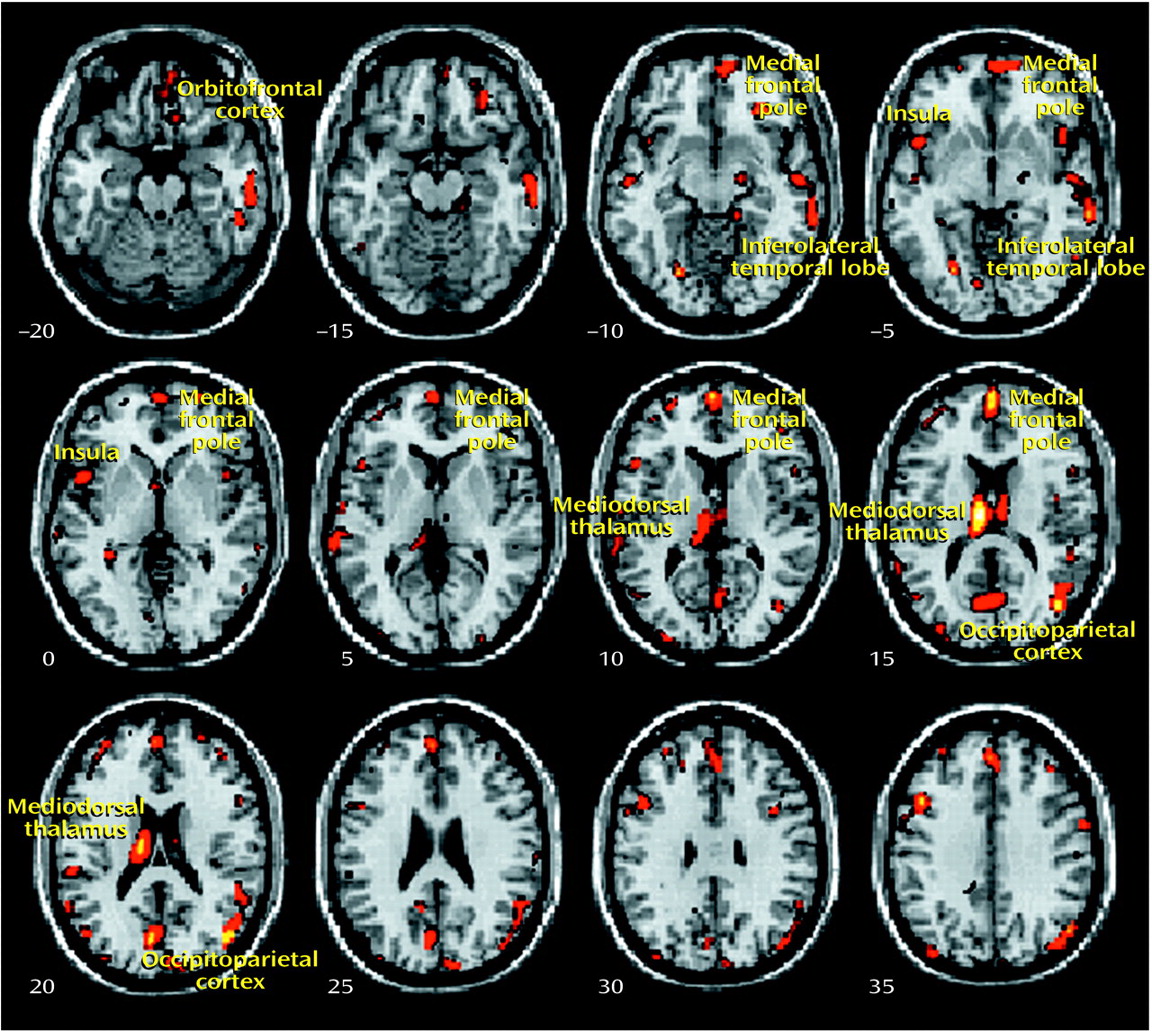
The present study used an optimized version of the voxel-based morphometry method to identify and characterize differences in brain structure associated with schizophrenia. Consistent with previous reports
(4–
8), we observed gray matter deficits and higher CSF volumes in schizophrenic patients. We also found regional differences in cortical and subcortical gray matter between schizophrenic patients and healthy comparison subjects, most significantly in the thalamus (especially the mediodorsal nucleus). These observations argue for thalamocortical dysfunction as a key component of pathoetiological mechanisms underlying symptoms and neuropsychological manifestations of schizophrenia.
The thalamus has previously been implicated in the pathoetiology of schizophrenia. Postmortem studies have reported neuronal loss in the maximal medial/mediodorsal thalamus of schizophrenia patients
(28,
29), and previous in vivo imaging studies have reported thalamic abnormalities in patients with chronic schizophrenia
(9–
12,
30), untreated schizophrenia
(31,
32), and childhood-onset schizophrenia
(20) as well as relatives of schizophrenic patients
(33) and schizotypal individuals
(34). In the meta-analysis of Wright and colleagues
(7), schizophrenia was associated with a mean 4% thalamic volume loss, with the proviso that only three studies contributed to this observed effect. Among studies that have failed to show thalamic volume differences between schizophrenic patients and healthy subjects
(35–
37), thalamic volume has been related to psychotic symptoms
(35). Functional imaging studies involving patients with schizophrenia also report functional abnormalities involving the thalamus, including abnormal thalamic activity at rest
(31), during auditory hallucinations
(38), and during attentional and executive tasks
(39,
40).
Our voxel-based morphometry findings add to the weight of evidence for structural abnormalities in the thalamus associated with schizophrenia. The conjunction of thalamic and distributed cortical gray matter depletion suggests that thalamocortical dysconnectivity may be crucial to the pathogenesis and symptoms of schizophrenia, consistent with specific theoretical models of cognitive integration and dysfunction in schizophrenia
(13,
41). Anatomically, the thalamus is a crucial relay between sensory pathways and cortex and also between sensory cortices and association areas. A proposed role of the thalamus as a sensory filter has been elaborated in relation to schizophrenia within the notion of “cognitive dysmetria”
(13,
15,
42), wherein the fluid coordination of mental activity is disrupted. Neuroanatomically, corticocortical pathways involving both the thalamus and cerebellum have been suggested to coordinate sensory processing with mental activity and behavioral sequelae
(15).
Dysfunction of coordinated regulation of sensory, motor, and cognitive processes may underlie abnormalities in sensory experience and attribution, motor coordination, and neuropsychological function spanning cognitive domains in schizophrenia
(3,
43). Within this model, abnormal filtering and gating mechanisms may result in disruption of synchronized signal processing (decreased signal to noise) within cortical association areas. The consequences of “mistimed information transfer” may account for hallucinations and delusions as misinterpretations of both external and internal percepts. Similarly, thought disorder may reflect uncoordinated language production, and negative dysexecutive symptoms may reflect deficits in associative processing of behavioral salience with sensory and mnemonic information. On this basis it has been argued that the integrity of thalamocortical connectivity, perhaps incorporating cerebellar mechanisms, is of primary pathoetiological importance in schizophrenia
(15). Moreover, alterations in functional dynamics of thalamocortical networks may disrupt mechanisms supporting more general conscious integration, thereby contributing to schizophrenic symptoms
(41). Our own findings of marked changes in thalamocortical architecture in schizophrenic patients add direct empirical evidence to support the theoretical proposal that functional abnormalities of this system underlie schizophrenia.
A key finding of our study was a demonstration of relationships between regional deficits in gray matter and clinical variables in schizophrenic patients. Although we observed a significant correlation between global reductions in gray matter and subjects’ age, which suggests progressive, accelerated neuronal loss in schizophrenia, the rate of gray matter decline with age of schizophrenic patients was equivalent to that observed in healthy comparison subjects. Moreover, there were no significant correlations between morphological changes and either age at onset of illness or years of neuroleptic treatment. These observations argue against a progressive involutionary disorder associated with schizophrenia. It is therefore of clinical and theoretical interest whether chemotherapeutic interventions aimed at halting the progress of “dementia praecox” will have an impact on cognitive symptoms and age-related gray matter loss in some individuals with schizophrenia.
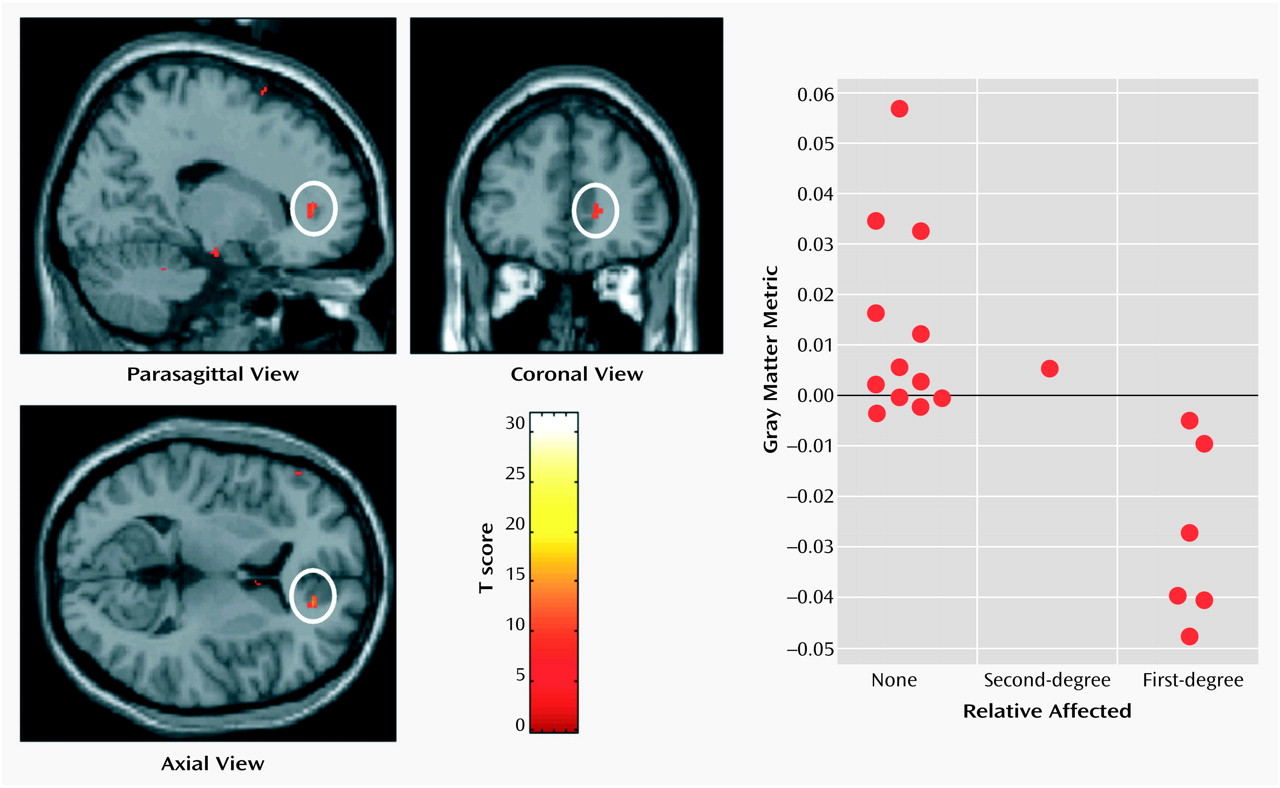
Thalamofrontal dysconnectivity may be particularly important in the pathoetiology of schizophrenia. Our observation that morphometric differences were most significant in the mediodorsal nucleus, which is reciprocally connected (and defines by its projection) to the prefrontal cortex, is noteworthy in that many of the cognitive deficits and symptom patterns manifested in schizophrenic patients are common to patients with prefrontal cortical lesions
(44–
46). Moreover, the medial prefrontal cortex demonstrated the strongest morphometric association with family history of psychosis, implying that genetic factors underlying maturation of prefrontal cortex and its connections may increase vulnerability to schizophrenia. Also, a higher level of educational achievement was associated with relative preservation of mediodorsal thalamic gray matter in schizophrenic patients, perhaps indicating greater integrity of thalamofrontal circuitry supporting executive planning and verbal reasoning. This is clinically important, since good premorbid functioning, suggested by educational achievement, is associated with reduced negative symptoms and more positive outcome in schizophrenia
(47).
A full understanding of the pathoetiology of schizophrenia remains an important goal, given the sociological and economic cost of maintaining services and therapeutic care for approximately 1% of individuals throughout the world. Recent technical advances, particularly in human genetics, molecular biology, and in vivo brain imaging, may lead to a more sophisticated understanding of biological mechanisms fundamental to mental health and disease. The present study used optimized voxel-based morphometry, which allows simultaneous, sensitive, and unbiased comparison of regional brain morphometry, thereby overcoming problems of region of interest hypothesis-driven analyses. Our evidence for thalamocortical gray matter depletion in schizophrenia, affecting distributed cortical regions, suggests a pathoetiological basis in re-entrant corticothalamic circuitry.