Major depressive disorder is a common, costly, and disabling condition affecting 4.9%–17.9% of the population in a lifetime. From 20%–30% of affected persons suffer a chronic, relapsing course. Many medications and several time-limited psychotherapies have shown established efficacy in randomized controlled trials. Among outpatients with major depressive disorder treated for the first time, about 50% will have a response (i.e., exhibit a clinically significant symptom reduction). However, of these “responders,” only 50%–70% will achieve symptom remission, which, since remission is associated with the best day-to-day functioning and best prognosis, is the goal of treatment.
For those cases of depression not remitting with the first treatment, little controlled trial evidence is available by which to select the next treatment. It is believed that a switch to a new, second treatment may result in a 50% response rate, with lower response rates expected with the third or fourth treatment step. Psychiatry faces the challenge of recommending the next best treatment steps, or series of steps, for depressed persons not experiencing a remission with the first or subsequent treatments. Therapeutic strategies may include switching (i.e., stopping one treatment and starting another) or augmenting/combining (i.e., adding a second treatment to the first).
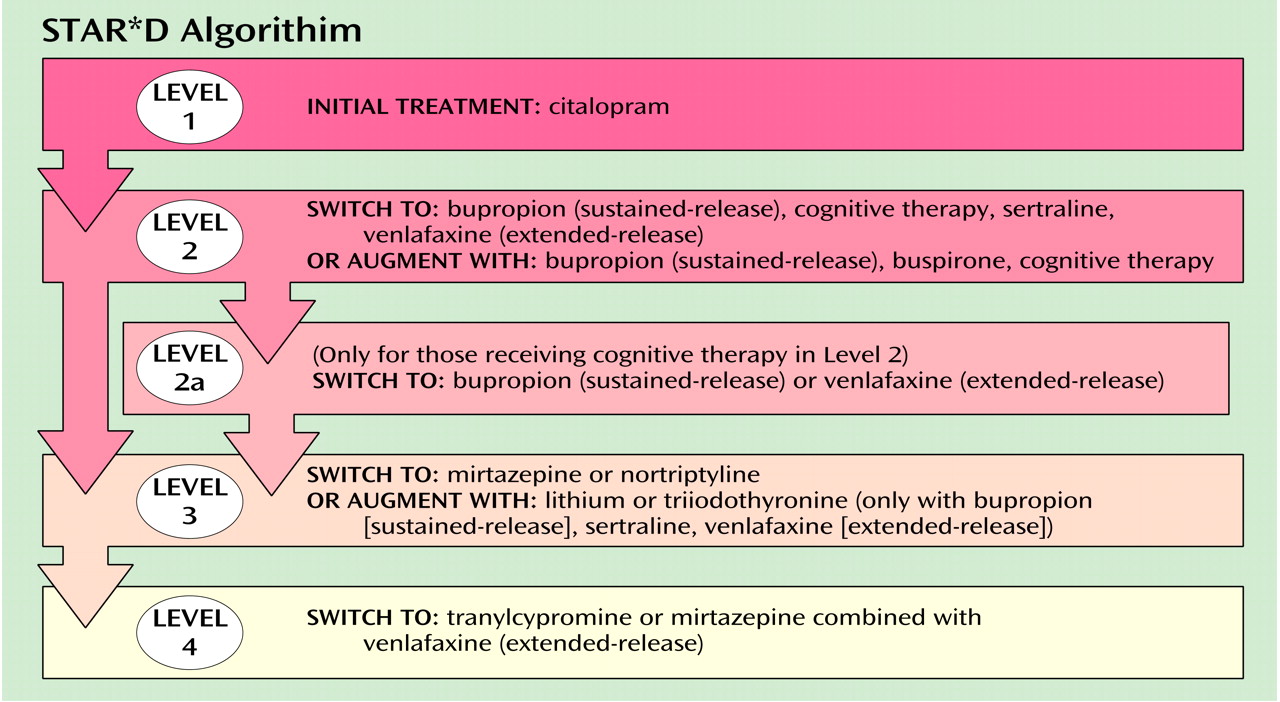
STAR*D (Sequenced Treatment Alternatives to Relieve Depression) is a multisite, prospective, sequentially randomized controlled trial of outpatients with nonpsychotic major depressive disorder that is using randomization to compare various switching or augmenting strategies (as shown in the Figure) either commonly used or that are based on pharmacologic reasoning. The study includes self-declared patients seeking treatment at either primary or specialty care practices.
Patients, as in practice, may select among the strategies. For instance, a patient may choose to only accept augmentation or only switching. However, all participants are randomly assigned to the specific treatments within the strategies that they find acceptable. This so-called equipoise stratified randomized design mimics clinical practice, so that results should have high practical relevance.
Response, remission, daily functioning, and service cost/utilization will be objectively assessed. Results, expected in the year 2006, will determine whether there is a preferred next step for varying types and degrees of treatment-resistant depression.