Neuroleptic-induced weight gain has been suggested to contribute to patient noncompliance with treatment and may adversely affect clinical outcome
(1). Obesity and being overweight are associated with reduced quality of life, greater morbidity (cardiovascular disease, diabetes mellitus, osteoarthritis, and some types of cancer), and mortality
(2).
Olanzapine, along with clozapine, has the greatest propensity of all available atypical antipsychotics to induce weight gain
(3,
4). It seems that most weight gain occurs early in treatment, and young patients previously unexposed to antipsychotic medication appear to be particularly vulnerable to olanzapine-induced weight gain
(5,
6).
The pathophysiological mechanisms underlying weight gain associated with atypical antipsychotic treatment have not been clarified. The adrenergic system may be relevant to iatrogenic weight gain. An increase in adrenergic tone (either through norepinephrine reuptake inhibition or stimulation of beta-3 or alpha-1 adrenergic receptors) has been associated with reduction of food intake, increase in energy expenditure, and weight loss
(7). The norepinephrine reuptake inhibitors phentermine and sibutramine, which inhibit both norepinephrine and serotonin (5-HT) reuptake, are potent appetite suppressants
(8). In contrast, atypical antipsychotics, primarily clozapine and olanzapine, exert an adrenergic antagonistic effect that may contribute—alone or together with their antagonistic effect at the 5-HT
2C and the histaminergic H1 receptors—to their high propensity to cause weight gain
(4,
9).
Reboxetine is a selective norepinephrine reuptake inhibitor, and its addition to conventional antipsychotics has been found to be safe and well tolerated in patients with schizophrenia
(10). We hypothesized that stimulation of adrenergic activity by the selective norepinephrine reuptake inhibitor reboxetine may diminish olanzapine-induced weight gain. In the present double-blind, placebo-controlled study, we sought to determine whether the addition of reboxetine would prevent or attenuate olanzapine-induced weight gain in patients with schizophrenia.
Method
Subjects and Study Design
Patients hospitalized at Tirat Carmel Mental Health Center (Israel) for DSM-IV schizophrenic disorder were enrolled in the study. Best-estimate diagnoses were based on information obtained from the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition
(11). Other inclusion criteria for the study were <4 weeks of antipsychotic drug exposure in the preceding 6 months, no previous olanzapine treatment, and a recommendation for olanzapine treatment by the treating physician during the current hospitalization. A double-blind, placebo-controlled, randomized design was used in the present study. The participants were allocated according to entries of a table of random numbers to receive a fixed dose of olanzapine (10 mg at 8:00 p.m.) with either reboxetine (4 mg/day [2 mg b.i.d.], N=13) or placebo (N=13) for 6 weeks.
Forty patients were screened for eligibility in the study. Fourteen patients were excluded for not meeting inclusion criteria (N=3), refusal to participate (N=4), uncooperativeness (N=2), aggressive or suicidal behavior (N=2), diabetes mellitus (which can affect body weight, N=1), and obesity (body mass index ≥30 kg/m2, N=2). Twenty-six patients (17 men, nine women) were included in the study. The olanzapine/reboxetine group consisted of 13 patients (nine men and four women; mean age=31.5 years [SD=12.9, range=19–46]; mean duration of illness=3.1 years [SD=3.0, range=0.5–10]; mean number of hospitalizations=1.2 [SD=0.3]). The olanzapine/placebo group also consisted of 13 patients (eight men and five women; mean age=30.0 years [SD=10.2, range=19–49]; mean duration of illness=2.8 years [SD=3.0, range=0.5–10.0]; mean number of hospitalizations=1.1 [SD=0.2]). Before the beginning of the study, three patients in each group were drug naive, and seven patients in each group had received haloperidol (5–20 mg/day). Two patients in the olanzapine/reboxetine group had received perphenazine (8–24 mg/day), and one patient in the olanzapine/reboxetine group and three patients in the olanzapine/placebo group had received risperidone (3–5 mg/day). None of the study participants received medications other than psychotropic agents. No abnormal findings were seen following a routine physical examination or in results of laboratory tests (which included an electrocardiograph and drug screening when appropriate).
The study was approved by the Tirat Carmel Hospital Review Board. Written informed consent was obtained from all participants after they received a full explanation of the nature of the study.
Administration of an anticholinergic agent (trihexyphenidyl, 5–10 mg/day) for extrapyramidal side effects and administration of a benzodiazepine (lorazepam, 1–3 mg/day) for insomnia or agitation were allowed on an as-needed basis, but no other antipsychotics, antidepressants, anxiolytics, or mood stabilizers were permitted. The doses of all medications remained unchanged during the entire study period. Meals were served three times a day, and patients were not placed on a special diet or physical exercise program for weight reduction.
Assessments
Body weight and body mass index were measured before breakfast at baseline and weekly afterwards. All weight measurements were performed by a research nurse who was blind to the patients’ treatment assignment. Clinical assessment instruments included the Scale for the Assessment of Positive Symptoms (SAPS)
(12), Scale for the Assessment of Negative Symptoms (SANS)
(13), Clinical Global Impression (CGI) scale for psychosis
(14), and the 17-item Hamilton Rating Scale for Depression
(15). Extrapyramidal side effects were assessed by using the Barnes Akathisia Scale
(16) and Simpson-Angus Rating Scale
(17). Emergent nonextrapyramidal drug-induced side effects were monitored through spontaneous reporting by the patients. Clinical ratings were completed at baseline and at week 6 by the same trained psychiatrist (I.I.), who was blind to the patients’ treatment assignments.
Statistical Analysis
Chi-square, two-tailed Student’s t test, and Pearson’s correlation analyses were used as appropriate. Six of the 26 study patients (three in each group) withdrew during the first week (before the second assessment) because of agitation that could have been related to the switch from typical antipsychotics to olanzapine. Since all dropouts were assessed only at baseline and no observation could be carried forward as a last observation, no intent-to-treat analysis was performed. Final statistical analysis included only those patients who completed the study (10 patients in each group).
Although weight and body mass index were measured weekly, we did not apply repeated-measures analysis, since the preliminary evaluation showed that for the mean values of both weight and body mass index, the trends over time were far from linear. In particular, there were no differences in the mean values of body weight and body mass index between weeks 2 and 3 or between weeks 4 and 5. Therefore, the mean changes in body weight and body mass index during the trial period (difference between baseline and each time point) were analyzed for each group. Analysis of the difference from baseline is equivalent to analysis of covariance with the coefficient for the covariate set at the value of one. Our preliminary analysis showed that for both weight and body mass index, those coefficients did not differ significantly from the value of 1 (mean=0.99 [SD=0.5] and 0.89 [SD=0.6], respectively). We also assessed the between-group difference in mean change from baseline for scores on the SAPS, SANS, CGI, and Hamilton depression scale. All results are expressed as mean and standard deviation. All p values are two-tailed at the significance level of 0.05.
Results
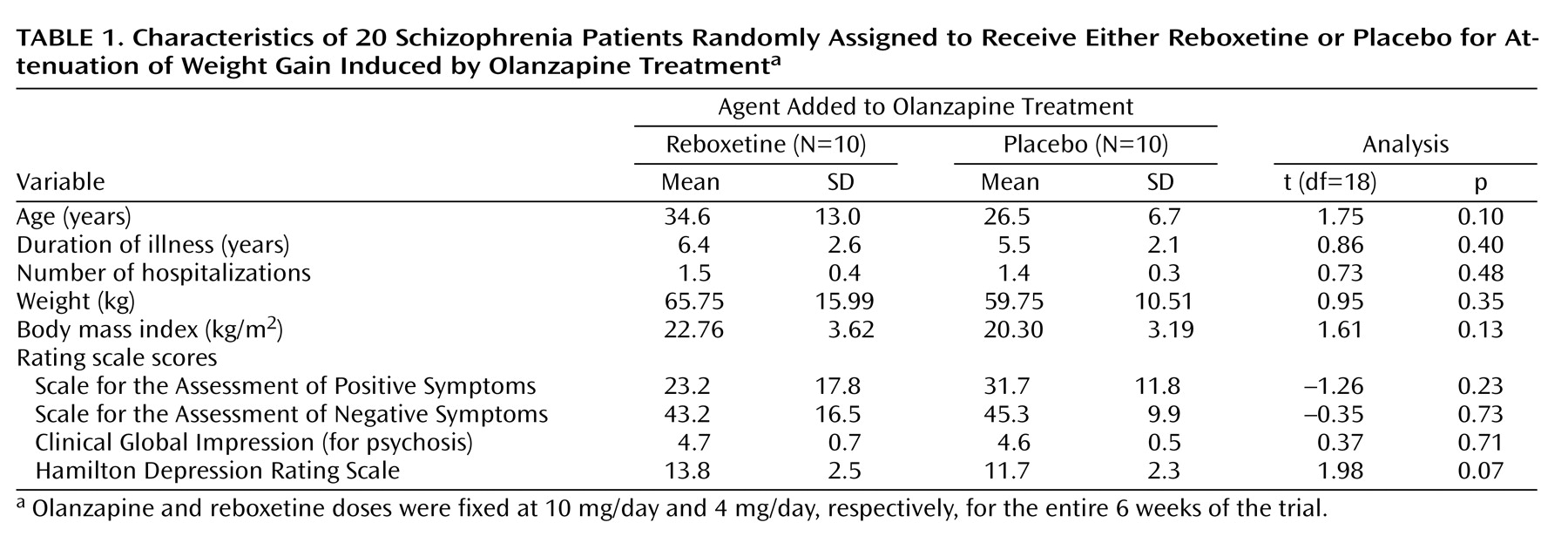
Twenty patients (10 in each group) completed the 6-week protocol. There were no differences in demographic or clinical characteristics or weight/body mass index values between the patients who dropped out of the study and those who did not. Each group consisted of six men and four women, and completers from the olanzapine/reboxetine group did not differ significantly from their olanzapine/placebo counterparts in clinical characteristics, baseline weight/body mass index values, and psychometric rating scale scores (
Table 1).
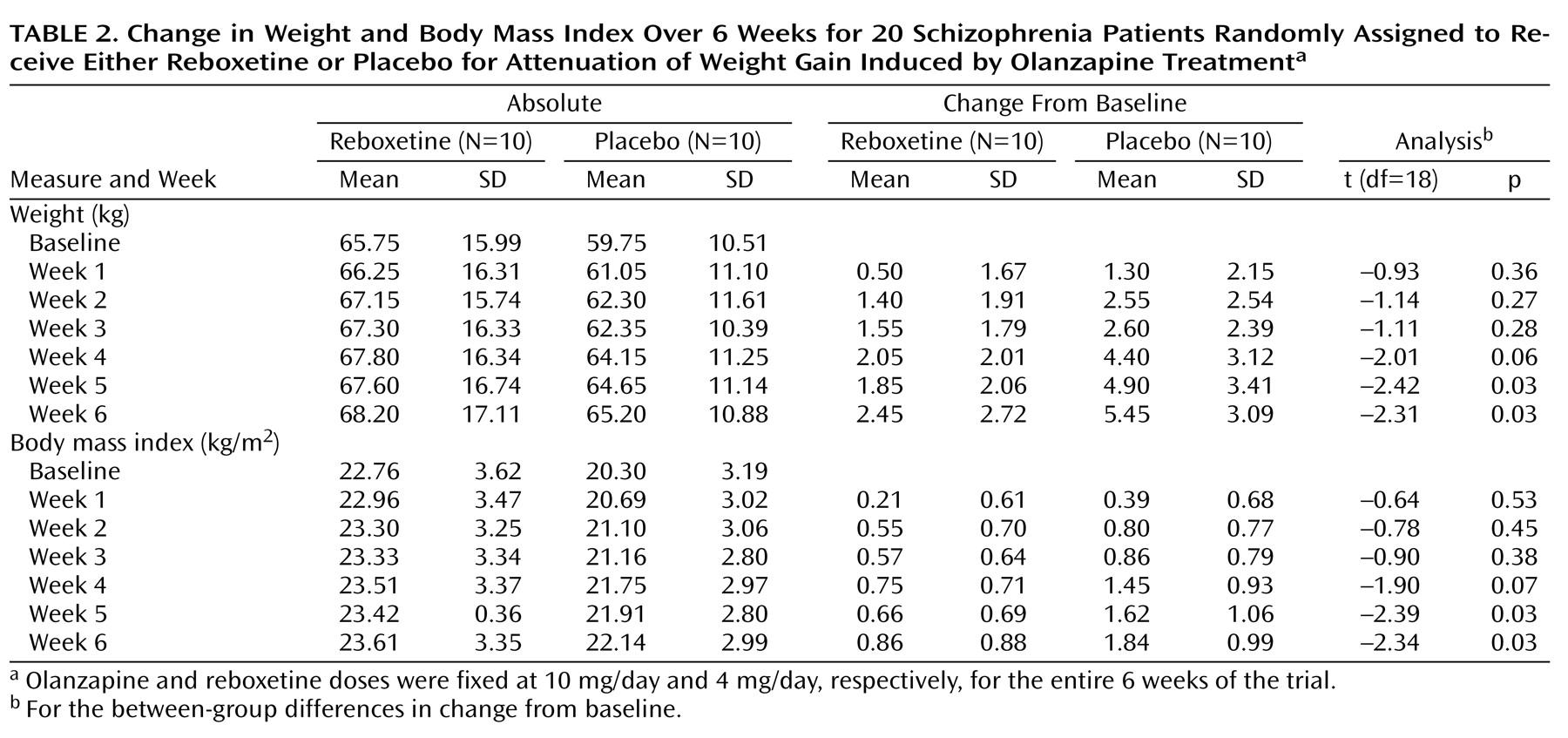
Body weight/body mass index changes from baseline in both groups are presented in
Table 2. Patients in both groups gained weight during the 6-week trial. However, at the end of the study, completers in the olanzapine/reboxetine group gained significantly less weight (mean=2.5 kg, SD=2.7, range=–0.5 to 7.5) than their counterparts in the olanzapine/placebo group (mean=5.5 kg, SD=3.1, range=0.5–11) (t=2.31, df=18, p<0.04), a mean between-group difference in weight gain of 3.0 kg (SD=1.3). Treatment-induced change in body mass index was significantly less in the olanzapine/reboxetine group (mean=0.86 kg/m
2, SD=0.88) than in the olanzapine/placebo group (mean=1.84 kg/m
2, SD=0.99) (t=–2.34, df=18, p<0.04), a mean between-group difference of 0.98 kg/m
2 (SD=0.42). It is noteworthy that the between-group difference in changes from baseline already emerged by the fourth week of the trial for both weight (mean difference=–2.35 kg, SD=1.17) and body mass index (mean difference=–0.7 kg/m
2, SD=0.37); these differences reached the level of statistical significance by the fifth week (
Table 2). Two of the 10 olanzapine/reboxetine-treated patients demonstrated weight loss, and one had no weight change, whereas all of the patients in the olanzapine/placebo group gained weight to some degree. Fewer patients in the olanzapine/reboxetine group (N=2 of 10) than in the olanzapine/placebo group (N=7 of 10) increased their initial weight by at least 7%, the cutoff for clinically significant weight gain
(18), which was a significant difference (χ
2=5.05, df=1, p<0.03). There was no significant correlation between initial body mass index and change in body weight at 6 weeks in either the olanzapine/reboxetine group (r=0.34, df=8, p=0.34) or the olanzapine/placebo group (r=0.02, df=8, p=0.96).
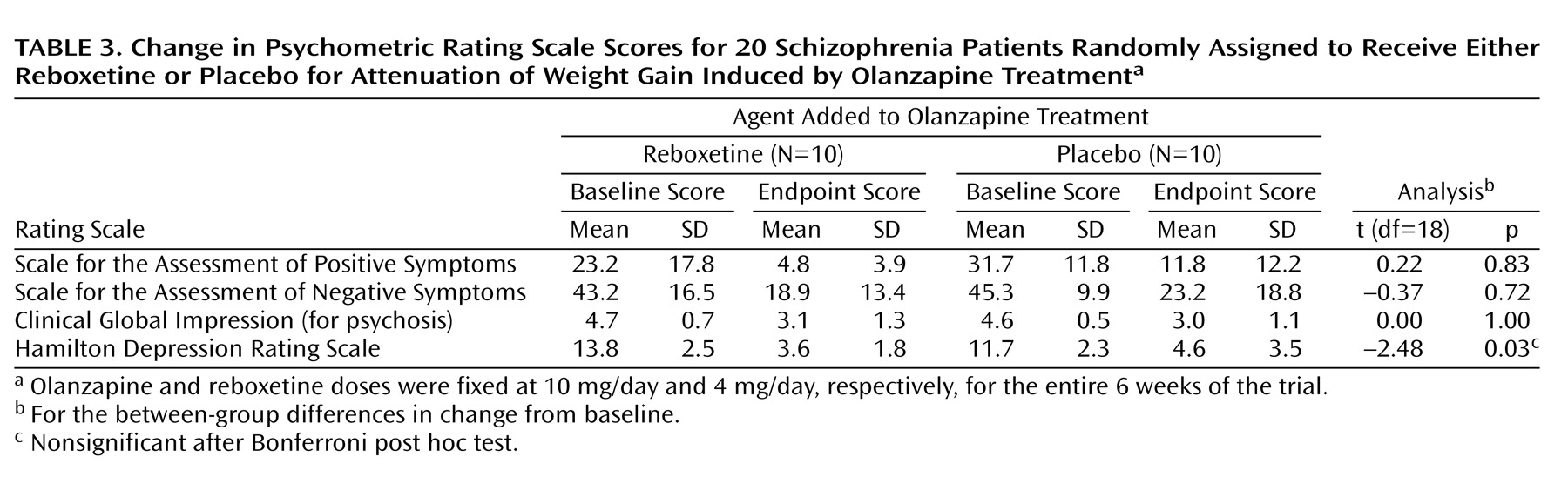
Patients from both groups demonstrated a significant reduction from baseline in scores of all psychometric rating scales (
Table 3). At the end of the 6-week trial, no between-group differences in change from baseline scores were revealed for the SAPS (mean difference=1.50, SD=6.95), SANS (mean difference=–2.20, SD=5.92), or CGI (mean difference=0.0, SD=0.43). The improvement in Hamilton depression scale scores was significantly greater in the olanzapine/reboxetine group than in the olanzapine/placebo group (mean difference=–3.10, SD=1.25).
The coadministration of olanzapine with reboxetine or placebo was well tolerated. Three patients in the olanzapine/reboxetine group and four in the olanzapine/placebo group had daytime somnolence. No treatment-induced motor hyperactivity was observed in either group during the study period. Mild akathisia (Barnes Akathisia Scale score of 2) was noted at baseline in five patients in the olanzapine/reboxetine group and three patients in the olanzapine/placebo group but disappeared in all cases by the end of the study. Parkinsonian symptoms (as measured by the Simpson-Angus Rating Scale) decreased in both groups; the between-group difference (mean=1.9, SD=2.03) was not significant (t=1.24, df=18, p=0.23). Anticholinergic medication for extrapyramidal side effects was not required by any of the study participants. Lorazepam (1–2 mg/day) for insomnia was required by 11 patients (six from the olanzapine/reboxetine group, five from the olanzapine/placebo group). No gastrointestinal side effects (e.g., nausea, vomiting, anorexia) were reported by the patients. No abnormal changes were noted in the results of routine laboratory tests.
Discussion
The present study confirms that olanzapine treatment is associated with substantial weight gain in schizophrenia patients. Moreover, our results are consistent with an earlier report that indicates that olanzapine-associated weight gain frequently occurs during the initial stage of treatment
(19).
The major finding of the present study is that the selective norepinephrine reuptake inhibitor reboxetine (4 mg/day) administered for 6 weeks attenuates olanzapine-induced weight gain in schizophrenia patients. Indeed, patients given olanzapine and reboxetine gained a mean of 2.5 kg (SD=2.7), whereas patients given olanzapine and placebo gained a mean of 5.5 kg (SD=3.1). It is of note that similar increases in body weight were observed in olanzapine/placebo-treated schizophrenia patients in our previous study of fluoxetine addition to olanzapine for attenuation/prevention of weight gain
(20). Finally, significantly fewer patients in the olanzapine/reboxetine group (N=2 of 10 [20%]) than in the olanzapine/placebo group (N=7 of 10 [70%]) increased their baseline weight by more than 7%, a cutoff for clinically significant weight gain.
The limitations of the present study are the small study group size, short duration of trial, and fixed doses of both olanzapine (10 mg/day) and reboxetine (4 mg/day). In addition, further studies should apply a pairwise matching for weight/body mass index to avoid between-group differences at baseline. In the present study, despite randomization, the olanzapine/reboxetine group had higher baseline weight/body mass index mean values than the olanzapine/placebo group. The differences, however, were not statistically significant. It is of note that these differences were mainly due to an outlier observation from the olanzapine/reboxetine group in which a subject with the highest weight/body mass index baseline values (92.5 kg and 25.09 kg/m
2, respectively) still exhibited the greatest weight gain in this group at the end of the trial (change in weight=7.5 kg; change in body mass index=2.04 kg/m
2). This observation and the lack of correlation between initial body mass index and change in body weight at the end of 6 weeks in both groups indicate that it is unlikely that the lower initial weight in the olanzapine/placebo group accounted for the greater weight increase. However, Kinon et al.
(5) demonstrated in a retrospective analysis of patients treated with olanzapine for at least 39 weeks that higher baseline body mass index was predictive of a lower long-term weight gain. It remains to be studied whether a longer duration of trial will be associated with a further weight-attenuating effect of reboxetine.
Reboxetine’s potential to attenuate olanzapine-induced weight gain may be explained by enhancement of norepinephrine neurotransmission through a selective blockade of the norepinephrine transporter. This notion is supported by evidence that both phentermine and sibutramine, two FDA-approved appetite suppressants, possess a potent inhibitory effect at the presynaptic norepinephrine transporter
(8). Unfortunately, these agents have not been examined in studies of neuroleptic-induced weight gain. Sibutramine, however, suppresses appetite and reduces weight by combined norepinephrine and 5-HT reuptake inhibition, leading to elevation of the availability of both monoamines in the hypothalamus
(21). It is of note that low potential to cause weight gain and even weight reduction have been reported in patients treated with the atypical antipsychotic ziprasidone, an agent with a unique potent antagonistic effect at both the norepinephrine and 5-HT transporters, in contrast to olanzapine and other atypical antipsychotics
(22). The relevance of the 5-HT transporter inhibition to attenuation of olanzapine-induced weight gain remains unclear, since in our previous study addition of the selective serotonin transporter inhibitor fluoxetine (20 mg/day for 8 weeks) was found ineffective in diminishing olanzapine-induced weight gain in a younger population of first-episode schizophrenia patients with a shorter duration of illness
(20). Thus, it is possible that stimulation of the norepinephrine rather than the 5-HT system may contribute to the attenuation of olanzapine-induced weight gain. Particularly, reboxetine may counteract olanzapine’s antagonistic effect at the alpha-1 and beta-3 adrenoreceptors
(9), which may disrupt peripheral or central energy homeostasis, resulting in weight gain. It is of note that sedentary lifestyle has been associated with weight gain
(4,
23). It seems unlikely that reboxetine’s effect on patients’ activity in the ward accounted for the observed diminished olanzapine-induced weight gain, since no between-group differences were revealed in either treatment-induced somnolence/sedation or hyperactivity. In addition, since in the present study we did not assess caloric intake (caloric counts, meal refusal, etc.), future studies should monitor the balance between caloric intake and caloric expenditure in an attempt to clarify the mechanism underlying reboxetine’s attenuating effect on olanzapine-induced weight gain. Finally, it seems unlikely that the significantly lower weight gain in the olanzapine/reboxetine group versus the olanzapine/placebo group was related to pharmacokinetic interactions between the two agents. Both agents exhibit only modest effects on hepatic cytochrome enzymes
(24,
25), and, to the best of our knowledge, there are no published data on clinically relevant olanzapine-reboxetine interactions.
On the clinical level, reboxetine addition was safe and well tolerated and did not interfere with the antipsychotic effect of olanzapine. In contrast, caution is advised when currently available appetite suppressants are administered concurrently with neuroleptic agents
(26). An additional clinically relevant advantage of reboxetine over established appetite suppressants such as phentermine and sibutramine is its antidepressive effect, as revealed by the reduction in the Hamilton depression scale scores in our olanzapine/reboxetine-treated schizophrenia patients when compared with their olanzapine/placebo-treated counterparts. It is of note, however, that the olanzapine/reboxetine superiority did not remain significant following correction for multiple comparisons (
Table 3).
In conclusion, the results of the present study indicate that the addition of the selective norepinephrine reuptake inhibitor reboxetine may attenuate olanzapine-induced weight gain in schizophrenia patients. Reboxetine coadministration was safe and well tolerated and associated with a modest beneficial effect on depressive symptoms in this patient population. To the best of our knowledge, this is the first study to demonstrate meaningful weight gain attenuation with reboxetine in olanzapine-treated patients. Determination of the optimal dose and time course of reboxetine efficacy in prevention/attenuation of neuroleptic-induced weight gain warrants further large-scale controlled studies in first- and recurrent-episode schizophrenia patients. Since olanzapine doses greater than the 10 mg/day administered in the present study are common in clinical practice, evaluation of reboxetine’s impact on weight in a broader range of clinically relevant olanzapine doses merits further investigation. A comprehensive biochemical workup (e.g., cholesterol, lipid profile, insulin resistance, glucose level) is also needed to clarify whether the diminution of weight gain achieved by reboxetine addition to olanzapine is accompanied by improvement in patients’ “metabolic fitness”
(8).