Several theories postulate regional brain dysfunction in cortical and subcortical regions associated with affective disorders
(1–
3). Few studies have followed a patient group with imaging before and after treatment with a single antidepressant.
The imaging data thus far
(1,
2) suggest that abnormalities in regional cerebral blood flow (rCBF) accompany depression and are altered by treatment. We used [
99mTc]hexamethylpropyleneamine oxime single photon emission computed tomography ([
99mTc]HMPAO SPECT) to test the hypothesis that treatment with the novel antidepressant venlafaxine is associated with rCBF changes in limbic and frontal regions. Venlafaxine has a main action at serotonergic and noradrenergic receptors
(4).
Method
Permission for the study from the local research ethics committee and the U.K. Administration of Radioactive Substances Advisory Committee was obtained. After complete description of the study to the subjects, written informed consent was obtained.
The inclusion criteria were an ICD-10 diagnosis of depressive episode, an entry score on the Hamilton Depression Rating Scale
(5) of 18–30, no previous history of depression, and medication-free status at study entry. The exclusion criteria were comorbid psychiatric disorder, physical disorder, substance misuse, and pregnancy or breast-feeding. Seven patients with major depression, two men and five women, were recruited.
Baseline and posttreatment SPECT scans were performed. The patients were medication free for at least 2 weeks before the baseline scan. After the baseline scan each subject received venlafaxine, 37.5 mg b.i.d., for 6 weeks. A second scan was then performed. The 21-item Beck Depression Inventory
(6) and 17-item Hamilton depression scale
(5) were applied at entry and 6-week follow-up by a single rater (J.D.).
Cerebral blood flow imaging was carried out by using 99mTc HMPAO SPECT. The scanning was performed with a Camstar XRT gamma camera (GE Medical Systems, Waukesha, Wis.) with Nuclear Diagnostics software (Nuclear Diagnostics AB, Stockholm) and a 128×128-pixel matrix (resolution full width at half maximum, 12 mm). The scans took 32 minutes and proceeded in a 360° circular arc of 64 positions from an anterior projection around the head, with 30 seconds per angle, commencing 2 minutes after intravenous injection of 500 MBq of [99mTc]HMPAO. The scanning was performed in a consistent, quiet environment at rest. The gamma emissions from the patient were subject to low-energy general-purpose collimation before detection by the gamma camera. Brain images were computed in the oblique transaxial, sagittal, and coronal planes.
The scans were transferred to a workstation and processed by using SPM 99 statistical parametric mapping image analysis software (Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.bpmf.ac.uk/spm).
The scans were spatially normalized to the positron emission tomography (PET) CBF template provided with SPM 99 by using the option for linear spatial normalization. The spatially normalized scans were smoothed by using an isotropic gaussian spatial filter with a resolution of 12 mm full width at half maximum before the voxel-wise statistical analysis. Normalization of global voxel intensity was achieved by using proportional scaling.
The scans were statistically analyzed by using the appropriate SPM 99 module
(7). The baseline scans were compared vowel-wise to the posttreatment scans by using the statistics option “multisubject, different conditions.”
Results
The seven subjects (two men, five women) were all right-handed. Their mean age was 43 years (SD=9, range=33–60). All but two of the patients had been drug free for at least 1 year. Their mean pretreatment scores on the Hamilton depression scale and Beck Depression Inventory were 25.00 (SD=4.24) and 24.71 (SD=1.38), respectively, and the posttreatment Hamilton and Beck scores were 6.29 (SD=2.50) and 3.28 (SD=0.76), respectively. The mean percentages of improvement in scores with treatment were 40% for the Hamilton depression scale and 52% for the Beck Depression Inventory (for each scale, z=–2.38, two-tailed p=0.02, Wilcoxon matched-pairs signed rank test). At 2-year follow-up no patient had relapsed.
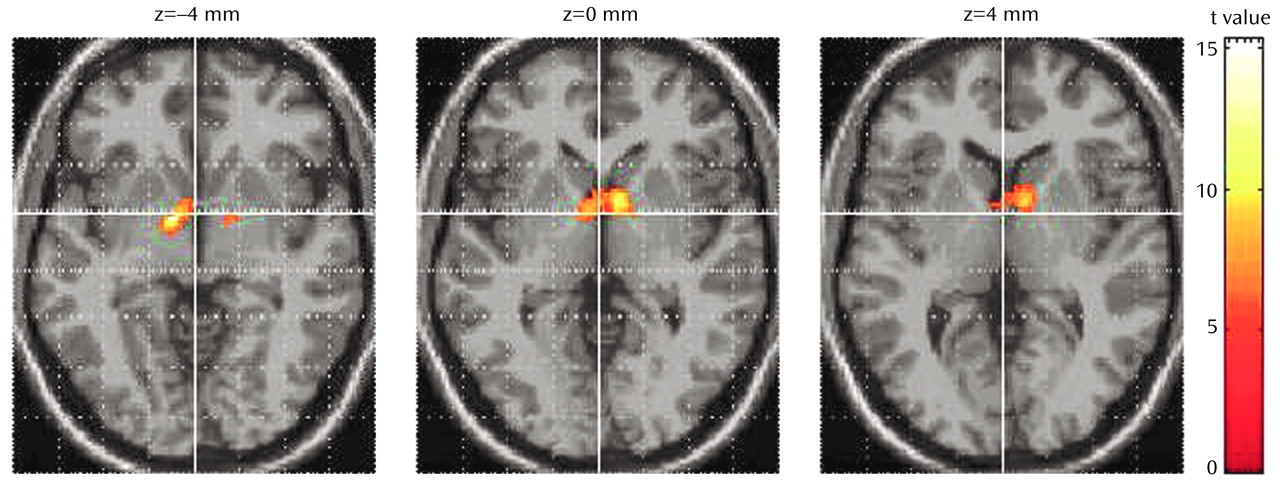
The results of statistical parametric mapping analysis showed a significant increase in blood flow after treatment in the thalamus bilaterally (
Figure 1). Significant rCBF decreases were also noted in the right posterior temporal lobe (Brodmann’s area 37), bilateral superior temporal gyrus (Brodmann’s area 21/22), left occipital lobe (Brodmann’s area 7), and right cerebellum. All results were thresholded at an uncorrected p<0.001 (for voxel height) and a corrected p<0.04 (for spatial extent).
Discussion
This is one of the first prospective studies confirming changes in cerebral blood flow after effective treatment with venlafaxine in drug-free, moderately ill subjects. Venlafaxine treatment resulted in clinical improvement in all cases. We have substantiated changes in the limbic system identified in previous studies
(8,
9).
PET and SPECT studies
(8,
10) have shown significantly lower baseline rCBF in patients than in healthy comparison subjects. In a PET study of venlafaxine for depressed outpatients
(11), baseline prefrontal and paralimbic hypometabolism was associated with antidepressant efficacy. Attempts to understand data from this field are complicated by many differences between studies, e.g., patient characteristics (severity, duration, and stage of illness), medication, and scanning techniques and instruments.
One explanation for the lack of significant changes in the frontal cortex in this study may be a type II error (false negative). The image-averaging technique used in SPM 99 is a 12-parameter affine transform that, while accounting for translation, rotation, size, and shape of the whole brain, does not make corrections for differences in individual gyri. SPM 99 software can correct for detailed differences in anatomy, but the correction is not satisfactory for images of limited resolution (∼12 mm). False negative results may result from the small number of subjects in this study. Nevertheless, important differences were found in distributed limbic and thalamic networks, and the possibility that moderate illness is not in fact associated with frontal dysfunction warrants consideration.
Identifying rCBF changes after successful treatment provides useful biological markers to guide discovery of novel drugs. Further studies in this area, with larger patient cohorts, are needed to identify blood flow patterns that predict response to treatment.