The use of concomitant psychotropic medication, defined here as the simultaneous use of two or more psychotropic medications (either for the same or different emotional/behavioral target symptoms), has been a common treatment practice in adults for a number of decades in many countries
(1,
2). The evidence clearly suggests that the use of concomitant psychotropic medication has been steadily increasing
(3,
4). This increase parallels the overall increase in the use of psychotropic medication, a phenomenon particularly prominent in the 1990s
(5,
6). In relation to individuals, concomitant psychotropic medication is prescribed more often to patients with the following characteristics and patterns: 1) a greater number and severity of comorbid disorders, 2) a greater number and severity of symptoms, 3) unsuccessful or partially successful medical treatment interventions, 4) visits to multiple physicians, 5) visits to physicians who are more prone to prescribe medications, 6) visits to psychiatrists, and 7) advancing age
(7,
8).
Although the use of concomitant psychotropic medication for youths has trailed the practice for adults, its use has expanded prominently since the late 1980s
(9–
11). Primarily because of the very limited scientific data on the use of concomitant psychotropic medication in youths, the practice has received little or no mention in child psychiatry and child psychopharmacology textbooks
(12–
16). Nonetheless, the very sizable increase in the use of concomitant psychotropic medication among youths merits a presentation and synthesis of the evidence on the use, efficacy, and safety of this practice.
Method
A MEDLINE search from 1996 through October 2001 was conducted and augmented by a review of references from relevant journals and textbooks. Many recent presentations at psychiatry and child psychiatry meetings that would not have been identified in MEDLINE were also reviewed. No studies of the use of concomitant psychotropic medication for youth were excluded on the basis of group size, study design, or outcome measures. The age range, as defined for this review, was 0–19 years old.
This review was structured according to three main objectives. The first was to describe the extent of use of concomitant psychotropic medication by examining patterns of use in terms of the prevalence, proportional rates, trends, and factors associated with variation in use. The second goal was to review the evidence base for the effectiveness of concomitant psychotropic medication. The final objective was to identify the known potential risks associated with the use of concomitant psychotropic medication for youths.
Concomitant psychotropic medication was assessed in terms of four aspects of use. The prevalence of use of concomitant psychotropic medication refers to the percentage of individuals who received concomitant psychotropic medication in a defined population of youths. By comparison, the proportional rate of use of concomitant psychotropic medication refers to the percentage of the total number of youths who received concomitant psychotropic medication in a treated group (e.g., psychiatric outpatients and/or inpatients). A concomitant psychotropic medication trend refers to a temporal change in the prevalence or proportional patterns of use. Variations in use of concomitant psychotropic medication are examined in relation to 1) physician specialty, 2) special populations, 3) physician-reported symptoms and behavioral features, and 4) type and frequency of concomitant psychotropic medication regimens.
The evidence base for the effectiveness of concomitant psychotropic medication is derived from clinical studies. A meaningful categorization of the evidence base for psychiatric treatment based on the relative strength of research has recently been proposed by Jensen et al.
(17). Concomitant psychotropic medication for youth is therefore presented as controlled double-blind research, open case series reports—with and without outcome rating measures—and descriptive case reports.
The evidence base for the risks associated with the use of concomitant psychotropic medication is far less extensive. The data comprise published case reports of adverse drug events
(18) from concomitant psychotropic medication use in youth. In this report, an adverse drug event refers to the occurrence of an undesired effect after the use of concomitant psychotropic medication.
Results
Proportional Rates and Prevalence
We were able to locate two national population assessments that measured use of concomitant psychotropic medication in U.S. children. One revealed that in 1997–1998, 24.7% of the representative physician office visits for youths in which a stimulant prescription was written were also associated with the use of concomitant psychotropic medication. This represented a fivefold increase over the 1993–1994 rate of 4.8%
(19). The other was based on national parent report surveys in 1987 and 1996 pertaining to medication use by children and adolescents. Olfson et al.
(11), by comparing these 1-year estimates, reported that the use of multiple psychotropic medications increased eightfold. For youths (younger than 19) who were given at least one psychotropic medication, the rate of receipt of additional psychotropic medication rose 2.5-fold (4.7% to 11.7%) over that decade. Outside the United States, a recent prevalence study from the Netherlands
(20) indicated that psychotropic therapy for youths is almost exclusively monotherapeutic. Published data on the rate of use of concomitant psychotropic medication in youths come mainly from selected groups, and the findings vary considerably by diagnosis and treatment setting. Medical records of youths from psychiatric outpatient and inpatient settings have been the source of data for most of these studies.
Rates by Diagnostic Profile
The majority of concomitant psychotropic medication studies have focused on youths with attention deficit hyperactivity disorder (ADHD). Rappley and colleagues
(21) found the proportional rate of use of concomitant psychotropic medication to be 20% among 2- and 3-year-old children who were enrolled for 15 consecutive months in a Medicaid insurance program in 1995–1996 and were diagnosed with ADHD (N=223). Ghuman et al.
(22), using data from 1995–1999, reported a concomitant psychotropic medication rate of 26% for 3- to 5-year-old children referred to a developmental disorder clinic who were diagnosed with ADHD and who received stimulant medication. In an older cohort of youths in a health maintenance organization (HMO) setting, use of concomitant psychotropic medication in 1997–1998 was identified in 21% of 5–12-year-old children who were diagnosed with ADHD and who were receiving stimulant medication
(23). A 1996 U.S. practice research network survey compiled by Zarin and colleagues
(24) found that 49% of 166 youths (aged less than 15 years) who were treated by private psychiatrists for ADHD were receiving concomitant psychotropic medication, with 12% of that total receiving three or more psychotropic medications.
Medical chart audits from specialty outpatient clinics in Massachusetts revealed relatively high rates of use of concomitant psychotropic medication. In 1990, additional psychotropic medication was prescribed for 47% of youths diagnosed with ADHD
(25) who were being treated with nortriptyline. A medical chart audit in 1991
(26) revealed the use rate of concomitant psychotropic medication to be 39% for youths diagnosed with obsessive-compulsive disorder (OCD). In some specialty clinics, even higher rates of concomitant psychotropic medication were observed. For example, Cohen et al.
(27) reported a use rate of concomitant psychotropic medication of 68% from 1992–1996 among youths with ADHD, which was similar to the 71% use rate of concomitant psychotropic medication that Biederman and colleagues
(28) found from 1991–1995 among youths with bipolar disorder.
Reports of use of concomitant psychotropic medication are also high among youths diagnosed with schizophrenia. In an early 1990s treatment program for youths with schizophrenia, Kumra et al.
(29) noted that the mean number of psychotropic medications prescribed for each applicant youth was 2.1.
On the basis of parent reporting from a group referred to a center for social learning disabilities in July through December 1997, Martin et al.
(30) reported a 29% rate of concomitant psychotropic medication among 109 youths (mean age=13.9 years) who had a pervasive developmental disorder. Similarly, in the early 1990s, Aman et al.
(31) mailed a survey to parents of autistic youths (a 53% response) and reported that 28% of the identified youths (median age=13 years) given psychotropic medication were receiving concomitant psychotropic medication.
Proportional Use by Treatment Setting
Concomitant psychotropic medication use findings based primarily on medical chart audits also vary considerably by treatment site and setting. In 1990, a systematic review of medical charts in community mental health centers (CMHCs) located in Maryland, New York, and Ohio revealed that 9%
(9), 11%, and 22%
(32), respectively, of treated youths were receiving concomitant psychotropic medication. More recently, medical chart reviews conducted in different CMHCs in Maryland found that concomitant psychotropic medication was prescribed for 21% of treated youths in 1994
(9) and 22% of treated youths in 1997
(33).
The rate of use of concomitant psychotropic medication has been consistently higher in inpatient than in standard outpatient psychiatric settings. In 1976–1977, 30% of 100 Canadian psychiatric inpatient youths received concomitant psychotropic medication
(34). In a 1991 medical chart audit from three hospitals, Kaplan and Busner
(35) similarly reported a use rate of concomitant psychotropic medication of 36%. A slightly higher rate of concomitant psychotropic medication of 42% was noted with 1994 data from seven youth inpatient units in Maryland
(9).
Trends
The use of concomitant psychotropic medication among youths increased substantially during the 1990s. For example, the rate of use of concomitant psychotropic medication rose 133% (9% to 21%) from 1990 to 1994 in four Maryland CMHCs
(9). In a national sample of physician office visits for youth (under age 18), the rate of combined antidepressant and stimulant use increased from 4% in 1994 to 29% in 1997, reflecting a sevenfold increase
(36). Rushton and Whitmire
(10) noted a similar increase from 1992 through 1998 for combined stimulant and selective serotonin reuptake inhibitor (SSRI) treatment among youths aged 1–19 years. Although most rates of concomitant psychotropic medication for psychiatric clinic youths in the early and mid-1990s were below 50%, several posters presented in 2001 at national psychiatric meetings showed an increase in this respect to over 50%
(37–
39).
An important trend to note is that the higher rate of use of concomitant psychotropic medication among youths has occurred simultaneously with the overall increase in psychotropic use. On the basis of findings from an HMO and two state Medicaid databases covering 1987 through 1996, the prevalence of psychotropic use among youths less than 20 years old increased 4–10-fold for antidepressants, 36–153-fold for alpha agonists, and three- to sevenfold for stimulants
(40). Likewise among youths (younger than age 20) in one state Medicaid program from 1996 through 2000, there was a threefold increase in neuroleptic treatment
(41). The increase was most prominent after atypical neuroleptics were introduced in the early 1990s
(42).
Variations
Physician specialty
Psychiatrists prescribe far more concomitant psychotropic medications for youths than do primary care physicians
(43,
44). This relates primarily to the fact that psychiatrists treat comparatively more seriously disturbed children
(43,
44). In all likelihood, the severity of the disorder also explains the higher rates of use of concomitant psychotropic medication in psychiatric specialty clinics, in which more severely impaired youths are seen than in CMHCs.
Besides the expected variation by physician specialty, prominent variations in concomitant psychotropic medication and psychotropic medication prescribing have been reported for individual physicians. Ahsanuddin et al.
(34), for example, noted that the use rate of concomitant psychotropic medication varied from 0% to 66% among five individual child psychiatrists.
Special youth populations
Youths with a greater degree of emotional, educational, or social impairment are more likely to receive concomitant psychotropic medication. Special populations whose use of concomitant psychotropic medication has been noted include youths in special education classrooms, in foster care, and in residential treatment centers. Mattison
(45) reported that 17% of 84 elementary school students in a special education classroom for children with emotional disorders were receiving concomitant psychotropic medication in 1993–1994. Bussing et al.
(44) similarly found the use rate of concomitant psychotropic medication to be 20% among elementary school students in a special education program (N=102) in 1995. Zima et al.
(46) reported that 45% of foster care youths aged 6–12 years who were taking psychotropic medication in 1996–1998 (N=38) were receiving two or more psychotropic medications. Likewise, Anderson et al.
(47) reported that 52% of a consecutive group of Illinois children in state legal guardianship (wards of the state) who were given psychotropic medication received more than one type concomitantly in 2001. Connor and colleagues
(48) assessed use of concomitant psychotropic medication during 1991–1992 in a Massachusetts residential treatment center and found that the rate was 57%–60% from the youth’s medication history and 40% at the time of admission.
Major Symptoms and Behaviors
The use of concomitant psychotropic medication has been found to be highly associated with aggressive behavior in youths, particularly the coadministration of neuroleptic medications
(32,
49). Ahsanuddin et al.
(34) noted that aggressive behavior disorders were the primary diagnoses associated with the use of concomitant psychotropic medication. Likewise, in a study of youths in residential treatment, Connor et al.
(48) found that the major symptom pattern associated with use of concomitant psychotropic medication was aggressive behavior.
Insomnia is another target symptom associated with the use of concomitant psychotropic medication in youths. Wilens et al.
(50) and Prince et al.
(51) both reported that clonidine is frequently added to stimulant treatment to aid sleep. In recent years, risperidone has been added to other psychotropic medications to both aid sleep and lessen aggressiveness
(52).
Common Combinations
A particularly common concomitant psychotropic medication combination for youths has been methylphenidate and clonidine. In the early 1990s, Swanson et al.
(53) estimated from a national pharmaceutical market source that 41% of surveyed youths in 1994–1995 who were receiving clonidine were also receiving methylphenidate. Prince et al.
(51), in a psychiatric clinic survey from 1992 to 1995, reported the rate of this combination to be 68%. In a 2–3-year-old Medicaid population, Rappley et al.
(54) similarly found this to be the most common psychotropic combination.
Another common concomitant psychotropic medication prescribing pattern involves antidepressants with stimulants. Pathiyal et al.
(55), in an 18-month longitudinal analysis of pharmacy claims data, reported that 22% of youths receiving methylphenidate in 1993–1995 also received antidepressants concomitantly. On the basis of 1998 North Carolina Medicaid claims data, Rushton and Whitmire
(10) reported that 30% of youths receiving an SSRI in 1998 also had a stimulant prescription during the same year. Similarly, Zito et al.
(56) found that one-third of Medicaid youths in one state who were receiving antidepressant medication in 1994 had also been given a stimulant that year. It should be noted that these prevalence findings for a 1-year period may not reflect the use of concomitant psychotropic medication. However, Rushton and Whitmire
(10) reported that 83% of stimulant/SSRI prescriptions were filled in the same month, suggesting concomitant use.
Evidence of Effectiveness
Controlled clinical trials
There are a number of controlled studies of adults that support the use of concomitant psychotropic medication for psychotic depression
(57), treatment-resistant depression
(58), bipolar I disorder
(59), and OCD
(60). By comparison, the published controlled, double-blind studies of concomitant psychotropic medication in youth (listed in
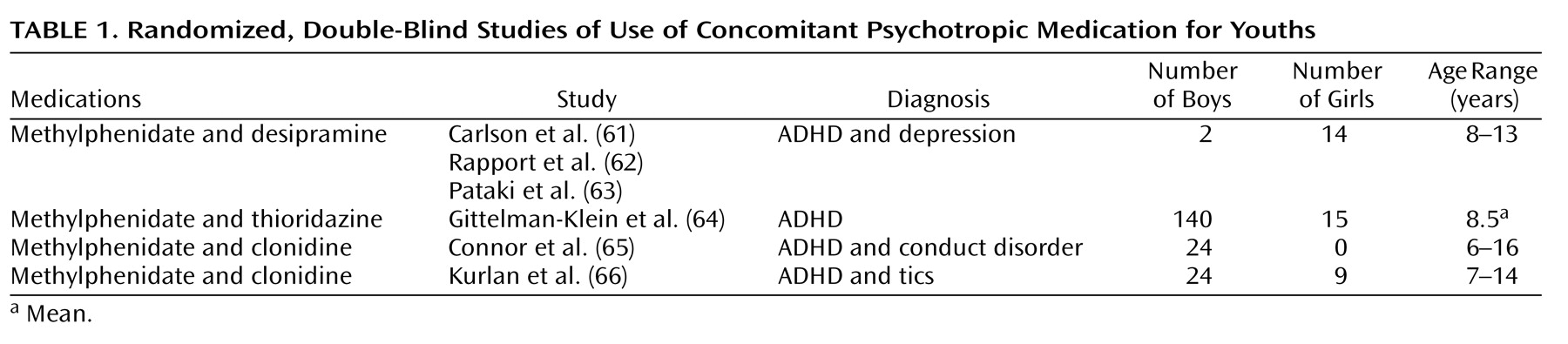
Table 1) are few in number. The first study
(61) evaluated a combination of methylphenidate and desipramine, which improved the clinical picture of ADHD to a modest degree over that of each drug individually. However, in this study of 7–12-year-old hospitalized youths, the combination resulted in impaired vigilance
(62) and more side effects
(63). Another study of youths (mean age=8.5 years) with ADHD
(64) noted that the combination of methylphenidate and thioridazine was rated as more satisfactory by parents—although not by teachers—than methylphenidate alone and thioridazine alone. However, the methylphenidate-thioridazine combination resulted in no greater overall clinician-rated improvement than methylphenidate alone and produced more side effects. A 3-month pilot study involving 24 youths aged 6–16 years with ADHD and an aggressive behavior disorder
(65) measured the behavioral effects of clonidine alone, methylphenidate alone, and the combination of the two. On all measured indices, the combination of clonidine and methylphenidate (N=8) was found to be equal to or inferior to that of methylphenidate alone.
A recent report describing a double blind, placebo-controlled study of 136 youths (aged 7–17 years) with both ADHD and tic disorders
(66) found that clonidine in combination with methylphenidate decreased teacher-reported ADHD impulsivity and hyperactivity symptom ratings somewhat more than either drug alone. A study by Turgay et al.
(67) noted that in youths (aged 5–12 years, N=45) with a disruptive behavior disorder and a subaverage IQ, the combination of a stimulant and risperidone resulted in no additional symptomatic benefit over risperidone alone.
In a systematic, individualized, carefully evaluated, blind assessment of seven hospitalized youths diagnosed with both a mood and a disruptive behavior disorder, the addition of lithium to methylphenidate resulted in no significant benefits over methylphenidate alone, and the outcomes of the combination were, for the most part, inconclusive
(68).
Two double-blind, controlled studies (see
Table 1) assessed methylphenidate augmented with either thioridazine or desipramine. The two augmenting medications have been used less of late because of potentially problematic cardiac conduction side effects
(69,
70). Consequently, combinations of these add-on drugs would need to be initiated with an unusual degree of caution. Also, using concomitant psychotropic medication to augment stimulant medication for most youths with ADHD is usually unnecessary since evidence from the Elia et al. study
(71) indicated that stimulants at high doses can be effective in 91% of the patients selected for an incomplete stimulant response.
Augmentation
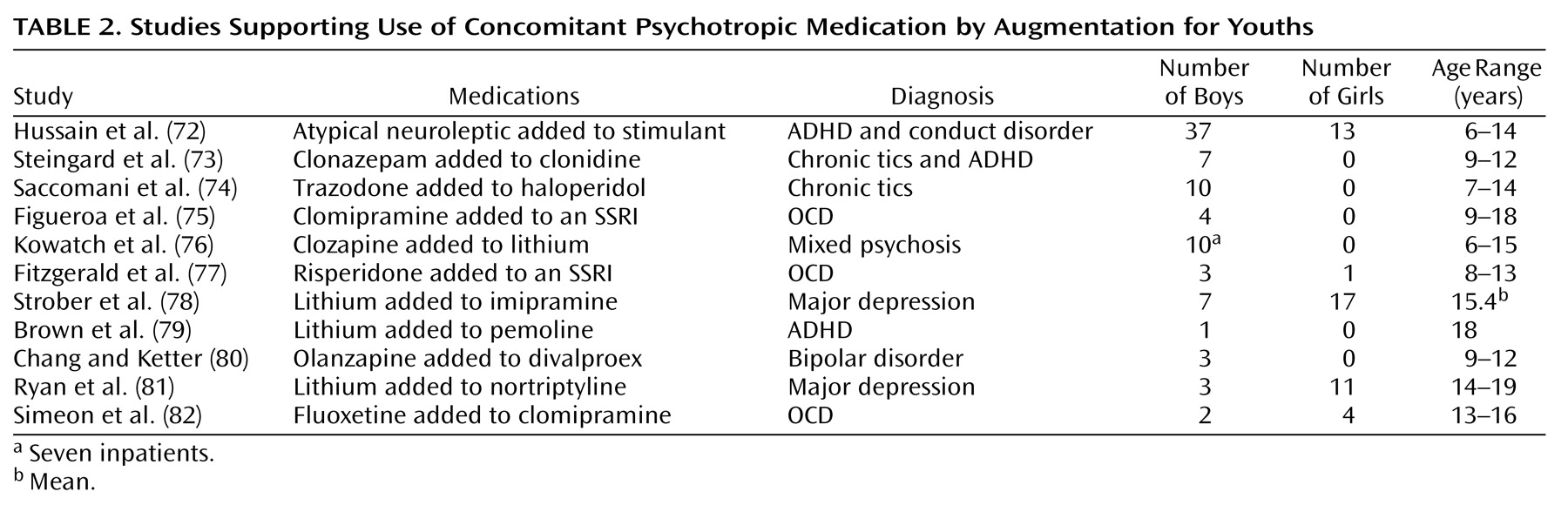
Studies that support the use of concomitant psychotropic medication for youths usually justify it in terms of using a second medication to augment an inadequate clinical response from the primary medication and treating comorbid psychiatric disorders. With the partial exception of the large study by Hussain et al.
(72), the concomitant psychotropic medication augmentation studies listed in
Table 2 are quite similar. They are generally small series, open reports covering up to 11 patients, with follow-up periods ranging from 1 to 48 months. Except for the study by Strober et al.
(78), all noted generally positive results. The patient outcomes are composed of either descriptions of individual outcomes (two reports) or average clinician- or patient-rated changes in target symptoms (nine reports). The Hussain et al. report
(72) was also open and uncontrolled, but it differed in that it covered 50 patients and contained independent teacher ratings.
Comorbid treatment
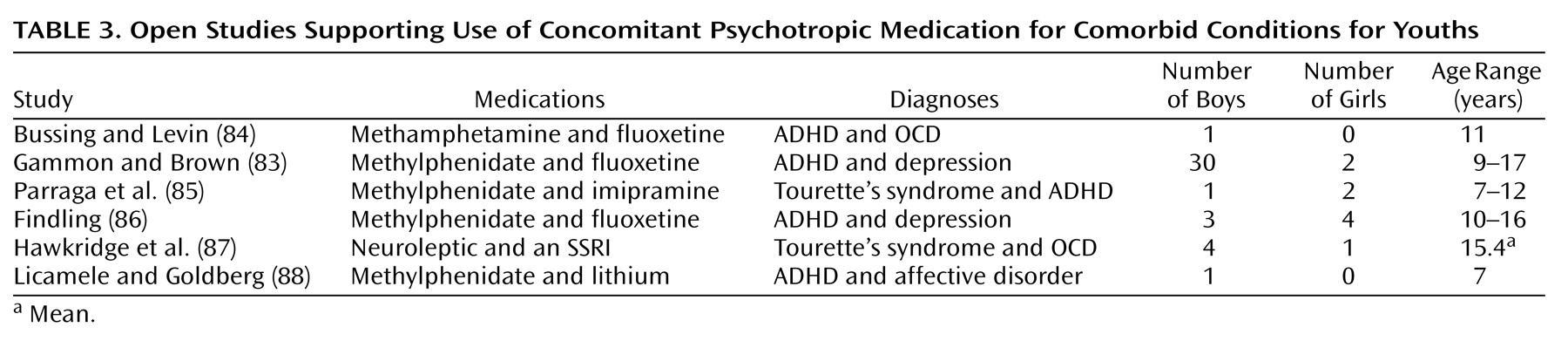
With the exception of the 32 patients in the study by Gammon and Brown
(83), the five other studies listed in
Table 3 covering the use of concomitant psychotropic medication for comorbid indications were small series and open studies covering up to seven patients. The evaluations were accomplished through the use of standard rating scales in five of the six studies; these were completed by clinicians (N=4) and/or by teachers (N=3). All of the reports covering the use of concomitant psychotropic medication for comorbidity were uncontrolled, and all concluded that the concomitant psychotropic medication was beneficial. It is noteworthy that in
Table 1,
Table 2, and
Table 3 over one-half of the case reports of the use of concomitant psychotropic medication involved prepubertal youths, a majority of male youths, and youths with the diagnosis of ADHD. Also of note is that all of the reports listed in
Table 2,
Table 3, and
Table 4 reflect research that the study by Jensen et al.
(17) would categorize as level C in scientific merit—on a scale from a high of A to a low of C.
Evidence of Associated Risks
A fundamental underlying concern with concomitant psychotropic medication for youths is the virtual lack of rigorous systematic research to support its use and to understand its potential risks
(99,
100). This and certain publicized cases of use of extreme concomitant psychotropic medication led the Council of the American Academy of Child and Adolescent Psychiatry
(101) to recommend judicious and cautious use of concomitant psychotropic medication for youths.
The cases of concomitant psychotropic medication cited by Wagner
(100), Sallee et al.
(89), and Behr
(102) of prepubertal youths receiving four to seven psychotropic medications concomitantly that were associated with adverse drug events understandably “raised eyebrows” in the field. In part, this was due to the consistent finding in the adult literature indicating that the risk of adverse drug events increases with the number of concomitant medications administered
(103,
104). Turner et al.
(105) and Martinez-Mir et al.
(104), evaluating pediatric inpatients, likewise reported a significant increase in adverse drug events in relation to the number of concomitant medications used.
Other safety concerns with use of concomitant psychotropic medication in youths include 1) the greater possibility of untoward drug interactions
(106,
107) and 2) the creation of drug-induced behavioral toxicity after the addition of another psychotropic medication—a consequence not often recognized as such, which can then lead to even more complex drug therapy to treat that side effect
(100).
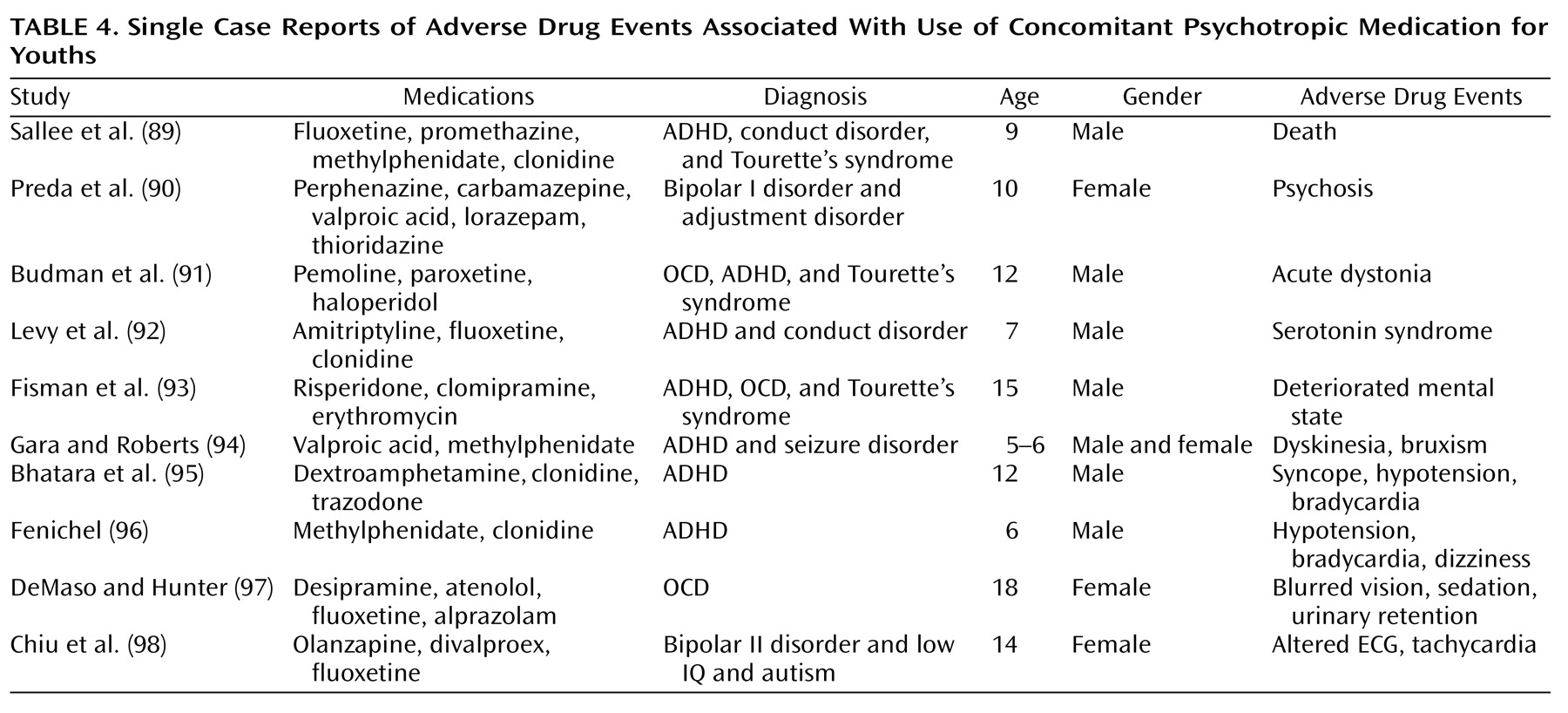
Table 4 presents a brief list of reports of adverse drug events with the use of concomitant psychotropic medication in youths. It is noteworthy that in nearly all cases, three or more psychotropic medications were administered concomitantly. The majority of the youths in these reports were boys younger than 13 years old.
Risks and Differences by Age
The risk of adverse drug events with the use of concomitant psychotropic medication in relation to age can be appreciated from data on valproate (used primarily for seizure control). From 1978 to 1984, the rate of fatal hepatotoxicity due to valproate monotherapy in youths under the age of 11 was five in 42,618. The rate of hepatic fatality when valproate was administered with one or more additional anticonvulsants increased prominently to 22 in 47,864, a fourfold increase. Moreover, during that survey period, there were no hepatic deaths among youths aged 11–21 years that were associated with valproate monotherapy, compared to five with valproate polytherapy
(108). One should also consider that, overall, youths aged 9 years or younger are substantially more at risk for adverse drug events than are older youths
(109).
Although differences in prescribing patterns in concomitant psychotropic medication in relation to age are incompletely studied, there are a number of findings in this area that are of interest. The use of concomitant psychotropic medication is more common with advancing age, in female adults, and in male youths
(110). Also, compared to adults, youths are prescribed different concomitant psychotropic medication combinations, have fewer multiple providers, and have different problematic behaviors targeted by concomitant psychotropic medication
(13,
110–112).
Discussion
Drawing inferences from the wide range of findings presented in this review with their modest generalizability is challenging. Several issues seem germane in considering the published concomitant psychotropic medication reports: namely, the rationale for concomitant psychotropic medication, U.S. academic perspectives, the emphasis on the treatment for aggression, drug and target symptom specificity, and the implications for better methods of assessment.
Rationale
Adding a second psychotropic medication to augment a partial response to the first could be useful if the first had been given at a customary therapeutic dose for a reasonable period of time and if that treatment and its subsequent augmentation received a systematically monitored assessment. The evaluation of the first medication and of the combination of medications should optimally be performed with reliable measures, baseline and subsequently rated targeted assessments, and preferably with at least one blind independent rater. “N of 1” research protocols for evaluating single patients in an experimental study might be developed to evaluate use of concomitant psychotropic medication more rigorously, beginning in teaching hospital settings under rigid research conditions and moving out to the community setting when methods are better developed.
Treating two comorbid psychiatric disorders with two or more different medications has been recommended if each of the disorders has a unique pattern and an optimal treatment. This rationale applies in certain instances (e.g., with tics and ADHD), but in most other circumstances, the diagnostic patterns are far less specific. In practice, there is often a sizable symptom overlap in comorbid diagnoses in child psychiatry (e.g., bipolar disorder and ADHD, disruptive behavior disorders and ADHD)
(28,
113). In addition, although a 100% treatment response would obviously be optimal, it is uncommon (particularly in chronic cases), and “doctors who cannot accept this level of imperfection put their patients at risk in a futile search for the holy grail through tactics like adding more drugs”
(114, p. 17). Green
(16, p. xi) similarly advised clinicians to “resist the tempting but erroneous notion that the right combination of drugs will solve any problem.” Furthermore, a number of investigators have reported successfully treating comorbid disorders with only one psychotropic medication by exploiting its range of effects, as has been shown by Klein et al.
(115), Spencer et al.
(116), Steingard et al.
(117), and Scahill et al.
(118).
Some writers in the field identify rational as opposed to irrational use of concomitant psychotropic medication
(119,
120). Essentially, a rational concomitant psychotropic medication approach is based on the weight of scientific evidence in its favor. However, as one notes from the available reports (
Table 1,
Table 2, and
Table 3), the evidence base for the use of concomitant psychotropic medication is profoundly limited. Consequently, the use of the term “rational” for the use of concomitant psychotropic medication applied to youths appears to be premature.
Academic Perspectives
Almost without exception, leaders in the field support use of concomitant psychotropic medication for specific indications in child psychopharmacology. Biederman
(121, p. 11) stated, “Examples of the rational use of combined treatment include the use of an antidepressant plus a stimulant for ADHD and comorbid depression, the use of clonidine to ameliorate stimulant-induced insomnia, and the use of a mood stabilizer plus an anti-ADHD agent to treat ADHD comorbid with bipolar disorder.” Popper
(122, p. 497) noted that “multiple agents are needed for treating comorbid presentations often seen in children, including ADHD with Tourette’s syndrome or obsessive-compulsive disorder (OCD), major depression with psychotic features, and certain depressive/anxiety disorders.” Although generally critical of many instances of use of concomitant psychotropic medication, Wagner
(100, p. 78) found it “very appropriate for co-morbid conditions in which there is a first-line treatment for each disorder.” She cited using an SSRI for major depression with a stimulant for ADHD as an appropriate example.
There have also been statements of support for specific combinations of concomitant psychotropic medications. For example, Carlson et al.
(123, p. 409) noted that “certain combinations appear to be useful.” These include 1) antidepressants with lithium for bipolar disorder, 2) lithium augmentation for refractory depression, 3) lithium and neuroleptics for acute psychoses, 4) lithium and anticonvulsants for refractory mania, 5) sustained-release methylphenidate with short-acting methylphenidate to increase potency at drug onset, and 6) methylphenidate and a late afternoon or evening neuroleptic for severely hyperactive children. Pliszka et al.
(113, p. 112) recommended use of concomitant psychotropic medication for youths with disruptive behavior disorders who are partially responsive to stimulant treatment, King
(124, p. 41S) recommended clonazepam or a low dose of a neuroleptic to augment the effects of SSRIs for youths with treatment-resistant OCD, and Emslie et al.
(125, p. 828) supported one of four psychotropic drug groups to augment inadequate medication responses to an SSRI for depressed youths.
Essentially, Biederman
(121) and Wagner
(100) promoted treating comorbid disorders as the major justification for the use of concomitant psychotropic medication in youths, whereas Carlson et al.
(123) recommended concomitant psychotropic medication to augment the treatment of partial medication responders. Others, such as Popper
(122), Theesen
(126), and Wilens et al.
(127), indicated that both augmentation and comorbidity justify the use of concomitant psychotropic medication in youths. Still other reasons to use concomitant psychotropic medication include allowing a lower dose of one agent to be used, reversing side effects, and alleviating symptoms while waiting for another medication to become effective
(16,
114,
120,
128).
Treating Aggressive/Disruptive Behavior
The admission of youths to inpatient psychiatric facilities in the United States is precipitated largely by aggressive/disruptive behavior
(34,
129–131). These youths receive concomitant psychotropic medication at a significantly higher rate
(34) and proportionally more neuroleptic medication
(32,
34,
49,
132) than nonaggressive youths. In fact, Connor et al.
(48, p. 27) wrote that “neuroleptics were used primarily for aggression regardless of diagnosis,” a finding previously noted
(32,
34). In addition to neuroleptics, psychotropic drugs used individually and concomitantly to treat aggressive behavior in youths included stimulants, lithium, antidepressants, anticonvulsants, and alpha agonists
(133).
Specificity of Drug Action and Symptoms
Child psychopharmacologists specifically aim to ameliorate discrete pathologically impairing target symptoms associated with diagnostic entities
(134). Generally, sleep impairment, enuresis, tics, depressive symptoms, hyperactivity, obsessive-compulsive features, intermittent impulsive behavior, and delusions are commonly targeted for reduction with psychotropic medication. The usual treatment approach is to focus on the reduction of one symptom or one symptom complex by using a low dose of one psychotropic medication initially
(13). However, when presented with an array of symptoms, one can easily extend this approach and add a different medication for each target symptom. A review of the adverse drug event reports comprising
Table 4 reveals that a very common aim of concomitant psychotropic medication in these patients had been to treat multiple target symptoms.
Implications for Better Assessment
Kutcher
(135, p. 257) noted that “in child and adolescent psychopharmacology, augmentation strategies are poorly researched, and in many cases, clinicians use adult literature as guidelines.” Sanchez et al.
(136, p. 639) similarly noted that most concomitant psychotropic medication for youths is “still based on studies in adults.” Extrapolation from adult studies to children and adolescents has numerous limitations because emotional disorders and psychotropic medication responses are often quite different in children and adolescents than in adults
(13,
137). Even mixing groups of late adolescents with younger youths can blur age-specific outcomes
(138). Likewise, concomitant psychotropic medication prescribing patterns for adults and youths prominently differ.
After reviewing the various methods for establishing evidence of efficacy, we propose that a systematic evaluation of concomitant psychotropic medication should take place on three levels. First, a national survey using probability sampling techniques can be conducted to estimate the prevalence and prescribing patterns of concomitant psychotropic medication across a spectrum of child and adolescent behavioral and emotional disorders as well as in different treatment settings, including primary care, psychiatry, and subspecialty practices. Second, the effectiveness, safety, and level of satisfaction with certain widely used drug combinations can be systematically evaluated by using multiple informants as well as standardized rating scales and questionnaires. Obtaining long-term outcome data on concomitant psychotropic medication in behavioral, academic, and social domains will, of course, depend greatly on the ability to retain subjects for extended periods. Third, patients receiving complex concomitant psychotropic medication could be addressed by using “N of 1” methods
(139). The methods for evaluating single patients in an experimental design originated in clinical psychology and randomly assigns the treatment interventions under double-blind conditions to successive time periods. The results of these trials generalize to the single patient under study; the goal is to produce an objective assessment of the benefit of the various interventions (drug 1 alone, drug 1 plus placebo, drug 1 plus drug 2). Applications of this approach have already been reported in the adult psychiatry and pediatric literature
(140,
141). If the approach is applied to concomitant psychotropic medication, careful monitoring of functioning and side effects could determine if the outcome is altered after a series of sequential evaluations of the concomitant medication or a placebo substitution. In regard to improving methods for assessing safety for rarely occurring events (less than 1 per 10,000 exposures), much work is needed. This will initially require an examination of weaknesses in the existing infrastructure for reporting adverse drug events and in the methods for producing evidence on risk assessment
(142,
143).
Comments
Drug combinations in general medicine in adulthood are a common, almost standard, practice for the treatment of certain conditions, such as seropositive HIV, intractable seizures, congestive heart failure, and hypertension
(144–
146). The level of pathology of these disorders can be monitored regularly, thus allowing medication treatments to be adjusted so as to reach or approximate the desired endpoint. In child psychiatry, although a sizable number of psychopathologic features are quantifiable, systematic monitoring in this regard is uncommon in clinical practice. Furthermore, the evidence base for the use of concomitant psychotropic medication, by comparison with adult practice, is quite weak
(17).
The trend for use of concomitant psychotropic medication in youths will probably increase at its present lively pace, far outpacing the capacity for better evaluation and assessment. Efforts in academic institutions to slow the use of multiple psychotropic medications have been tried and are usually partially successful. However, these results appear to be poorly maintained over time
(147–
149), a consequence due in all likelihood to an array of complex factors that continue to stimulate the use of concomitant psychotropic medication. These include a relatively weak response to the initial psychotropic agent, the influence of open studies reporting that concomitant psychotropic medication for youths is useful, the ready application of findings from adult concomitant psychotropic medication studies to youths, the failure to publish negative findings, a reluctance to gradually withdraw an add-on psychotropic medication that initially appeared to be beneficial, and the inability of many physicians to reduce a complex regimen that the previous clinician initiated. Added to these are the pressures for medication treatments from managed care organizations, direct-to-consumer and physician-directed pharmaceutical advertising, and distraught parents and beleaguered child care staff
(100,
150). Although this review cannot reverse the trend for the use of concomitant psychotropic medication in youths, it is hoped that it can aid in encouraging needed research in this area.