In the search for early risk factors related to schizophrenia, research has focused on the roles of predisposing genetic factors as well as on pregnancy and delivery complications. Monozygotic twins discordant for schizophrenia have been compared for possible early developmental differences
(1,
2), and it has been shown that the affected twins had more brain and neurological abnormalities
(3,
4). Increased risk of schizophrenia has also been linked with exposure to infections during the second trimester
(5), maternal stress
(6), and nutritional deficiencies in utero
(7,
8). Evidence is also accumulating for an association between obstetric complications and increased risk of schizophrenia
(9). Specific obstetric complications, such as preeclampsia, have been identified as an independent predictor of offspring vulnerability to the development of schizophrenia
(10). Since preeclampsia may impair fetal growth, it has been suggested that the etiopathogenesis of schizophrenia might involve a reduced nutritional supply to the fetus
(10). We have not been able to identify other studies that have further examined the relation between maternal preeclampsia and offspring vulnerability to schizophrenia, and there is a need to examine whether other maternal conditions related to preeclampsia, such as chronic hypertension, might confer an increased risk of the disorder in the offspring. It is also unclear whether medical treatment of obstetric conditions such as hypertension or preeclampsia might contribute to the etiology of schizophrenia. To our knowledge, no studies linking psychiatric morbidity in the offspring with specific treatment regimens in pregnancy have been reported, but research has demonstrated that diuretics prescribed after the first trimester because of hypertension interfere with normal plasma volume expansion and may cause volume depletion
(11). Thus, it is possible that prenatal exposure to diuretics and other antihypertensive drugs may disrupt neurodevelopment of the fetus.
Although diuretics have not been reported to have teratogenic effects, their use in pregnancy is controversial
(12). Homeostatic mechanisms are likely to protect the immature brain from the immediate effects of volume depletion during pregnancy, but it is theoretically possible that electrolyte imbalance or volume depletion after prolonged diuretic therapy may have delayed neurodevelopmental effects. The neurodevelopmental hypothesis of schizophrenia suggests that the disorder has its origin in aberrant brain development
(13). We hypothesized a link between schizophrenia in adult life and prenatal exposure to diuretics and/or maternal hypertension during pregnancy.
We undertook the present prospective study using data from the Copenhagen Perinatal Cohort to examine the associations between schizophrenia in adult life and prenatal exposure to diuretics and other pregnancy characteristics. Diuretics were rather commonly prescribed to pregnant women at the time the cohort was established
(14), and recent studies have shown that diuretics are still used for treating hypertension and cardiac diseases in pregnancy
(15). The possibility of linking the Danish Psychiatric Register
(16) with information on prenatal exposure to drug treatment in the Copenhagen Perinatal Cohort
(17) enabled us to conduct this study.
Method
Main Exposure Variables
The Copenhagen Perinatal Cohort consists of 9,125 individuals delivered from October 1959 to December 1961 at the maternity department of the Copenhagen University Hospital, Rigshospitalet. At the establishment of this cohort, demographic, socioeconomic, prenatal, and perinatal medical data were recorded prospectively. The data collection methods have been described in detail elsewhere
(14,
17,
18). The data on prenatal exposure to medication indicated class of substance (hormones, barbiturates, diuretics, antiepileptic drugs, etc.) and timing of exposure, by trimester.
Blood pressure was measured routinely during pregnancy at scheduled visits and documented in the medical records. Hypertension was classified as present if the blood pressure in more than one measurement was 140/90 or above, irrespective of whether the woman had previously been normotensive. Hence the definition used here most likely included both cases of chronic hypertension and cases of gestational hypertension. Hypertension was classified as either present or absent, and the current data set does not include ratings of severity. When the cohort was established, the variables of hypertension and preeclampsia were coded as mutually nonoverlapping categories. Preeclampsia was defined as the occurrence of hypertension, edema, and proteinuria after 20 weeks’ gestation and was classified as either present or absent. Preeclampsia arising in previously normotensive women could not with certainty be separated from preeclampsia arising in women with chronic hypertension. Treatment with diuretics and other drugs was recorded by trimester on the basis of a face-to-face interview a few days after the women had given birth. Of 424 women treated with diuretics at any time during pregnancy, two were exclusively treated in the first trimester, 15 in the second trimester, and 356 in the third trimester. Fifty-one women were treated in both the second and third trimesters. Thus, a total of 66 women were treated in the second trimester, and 407 in the third trimester.
Occurrence of Schizophrenia
The Danish Psychiatric Central Register was fully computerized by April 1, 1969
(16). It contains data on all admissions to Danish psychiatric inpatient facilities. The diagnostic system in use when the Danish Psychiatric Central Register was computerized was the ICD-8. In ICD-8, schizophrenia is defined by prototypic descriptions of symptoms, such as bizarre delusions, delusions of control, abnormal affect, autism, hallucinations, and disorganized thinking. In 1994, the ICD-10 classification was implemented. For this study, we identified all cohort members and their mothers who had been hospitalized with a diagnosis of schizophrenia before 1994 (ICD-8 codes 295.00–295.99). The risk set consisted of 7,866 individuals with data on both prenatal exposure to diuretics and data on psychiatric follow-up (3,994 men and 3,872 women).
Other Variables
Parental social status was recorded when the child was 1 year old according to an 8-point scale
(19). Data on social status were missing for 18.5% of the risk set. To avoid dilution of the data set, we replaced missing values for this variable with the variable mean. Social status was weakly associated with schizophrenia in the offspring, and since factors related to seeking medical treatment during pregnancy might be associated with social and educational variables, social status was a potential confounder. Although maternal age had little effect on the risk for schizophrenia in the offspring, this variable correlated with hypertension and diuretic treatment and thus might potentially confound the results. There was a strong association between a maternal diagnosis of schizophrenia and increased risk of the disorder in offspring. This potential confounder was included as a covariate. While prenatal exposure to barbiturates has been linked with long-term deleterious effects on cognitive performance
(18), no evidence has been found to link this exposure with schizophrenia. However, maternal hypertension was often treated with barbiturates, and consequently the effects of maternal barbiturate treatment were also controlled in subsequent statistical analyses.
Indexes of pregnancy complications and delivery complications have previously been developed by Danish and American obstetricians
(19). The index of pregnancy complications is a weighted index of specific events occurring during pregnancy. Events with a moderate effect include hypertension and jaundice during pregnancy. Events with a high level of effect include placenta previa, abruption of the placenta, laparotomy, and severe preeclampsia. The index of delivery complications is a weighted index of specific events occurring from the beginning of labor to the evaluation of the child’s condition at birth. These events include whether labor had been induced, whether the fetal presentation had been atypical, and whether delivery conditions had been “less than optimum.” Preliminary analyses showed nonsignificant relationships indicating higher indexes of pregnancy and delivery complications in preschizophrenic individuals (data not shown), and both indexes were used as summary evaluations of the course of pregnancy and delivery.
Data Analysis
We conducted chi-square tests of differences between cohort members with schizophrenia and cohort comparison subjects for the dichotomized main exposure variables. Variables approaching statistical significance (p<0.10) were included in multiple logistic regression analyses. First, the association between the exposure variables and the risk of schizophrenia was analyzed with adjustment for social status of the family breadwinner and the mother’s age. The second model estimated the influences of maternal hypertension and diuretic treatment during pregnancy on the risk of schizophrenia in the offspring while adjusting for maternal schizophrenia, parental social status, mother’s age, and prenatal exposure to barbiturates (the variables of parental social status and mother’s age were entered as continuous variables). In separate univariate analyses of variance, the distributions of potential confounders in hypertensive mothers who were prescribed diuretics late in pregnancy were compared with the distributions in untreated hypertensive mothers. In a separate analysis, the weighted indexes of pregnancy and delivery complications were included as continuous covariates to control for pregnancy and delivery complications. All analyses were conducted by using SPSS version 10.0 (SPSS Inc., Chicago).
Results
Eighty-four cohort members developed schizophrenia (1.1%).
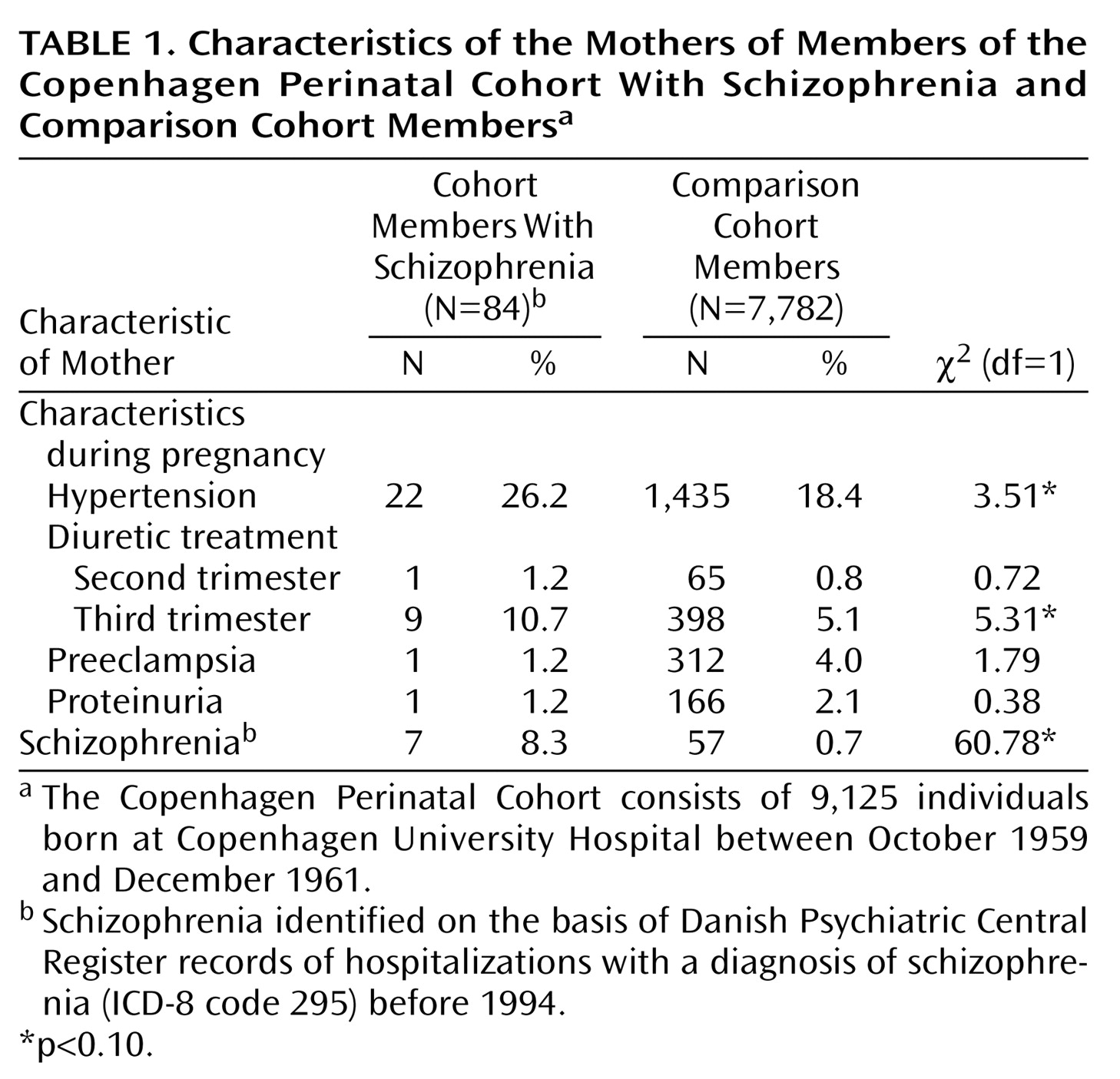
Table 1 shows the characteristics of the cohort members with schizophrenia and of the cohort comparison subjects. Univariate analysis showed that the two groups differed in the distributions of hypertension, diuretic treatment in the third trimester, and maternal schizophrenia (p<0.10). The distributions of maternal preeclampsia, proteinuria, barbiturate treatment in the second or third trimester, and diuretic treatment in the second trimester were not significantly different between groups.
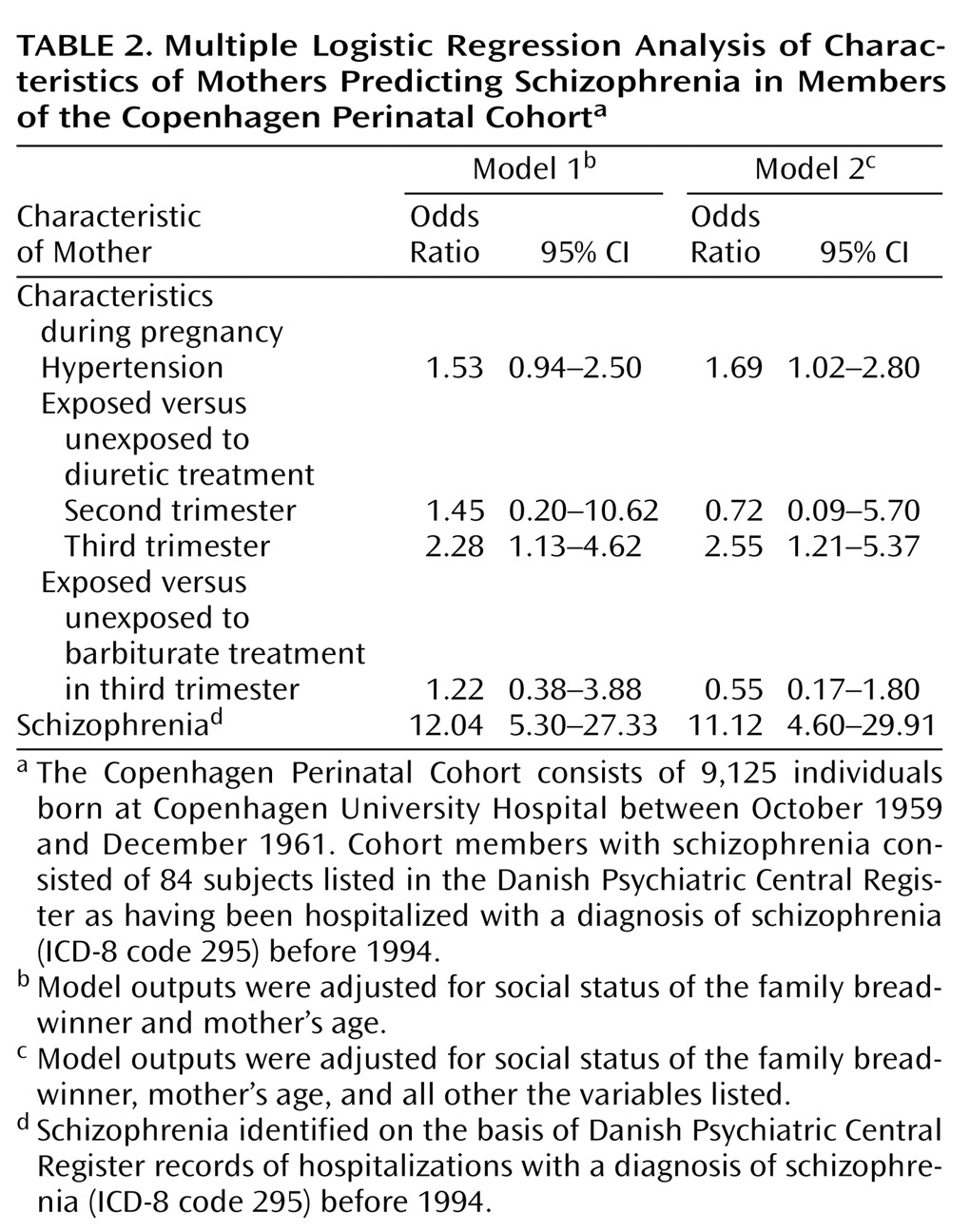
Table 2 shows the results for the multiple logistic regression analyses. Data in the first column indicate the effects of the variables listed, with the effects of maternal age and social status controlled. Increased risk of schizophrenia was strongly associated with having a mother with schizophrenia and less strongly associated with prenatal exposure to diuretic treatment in the third trimester. When effects of maternal schizophrenia, treatment with diuretics in the third trimester, and maternal hypertension were assessed jointly in the model (second column), all three factors emerged as independent predictors of schizophrenia. We did not find significant associations between prenatal exposure to diuretics and other psychoses besides schizophrenia. Neither prenatal exposure to diuretics nor maternal hypertension was associated with elevated risk of developing reactive psychosis (ICD-8 code 298), other psychosis (ICD-8 code 297), or affective psychosis (ICD-8 code 296).
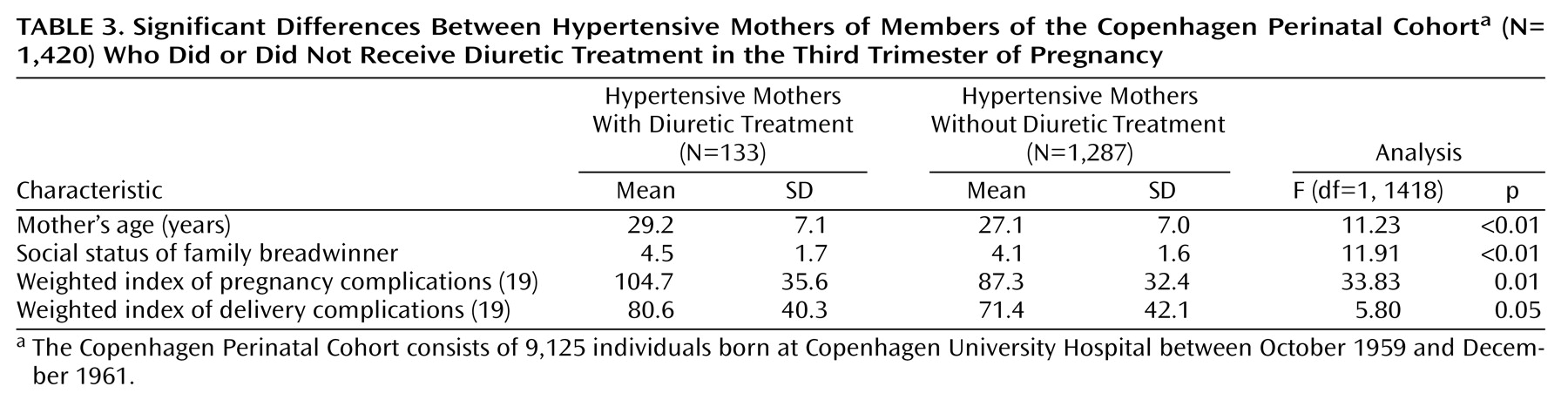
Diuretics were prescribed in the third trimester to treat hypertension, among other conditions. Presumably, the mothers with more severe cases of hypertension received treatment, while those with milder cases did not. Univariate analysis (
Table 3) showed that the distributions of maternal age, social status, and indexes of pregnancy and delivery complications differed significantly between diuretic-treated hypertensive mothers and untreated hypertensive mothers. As shown in
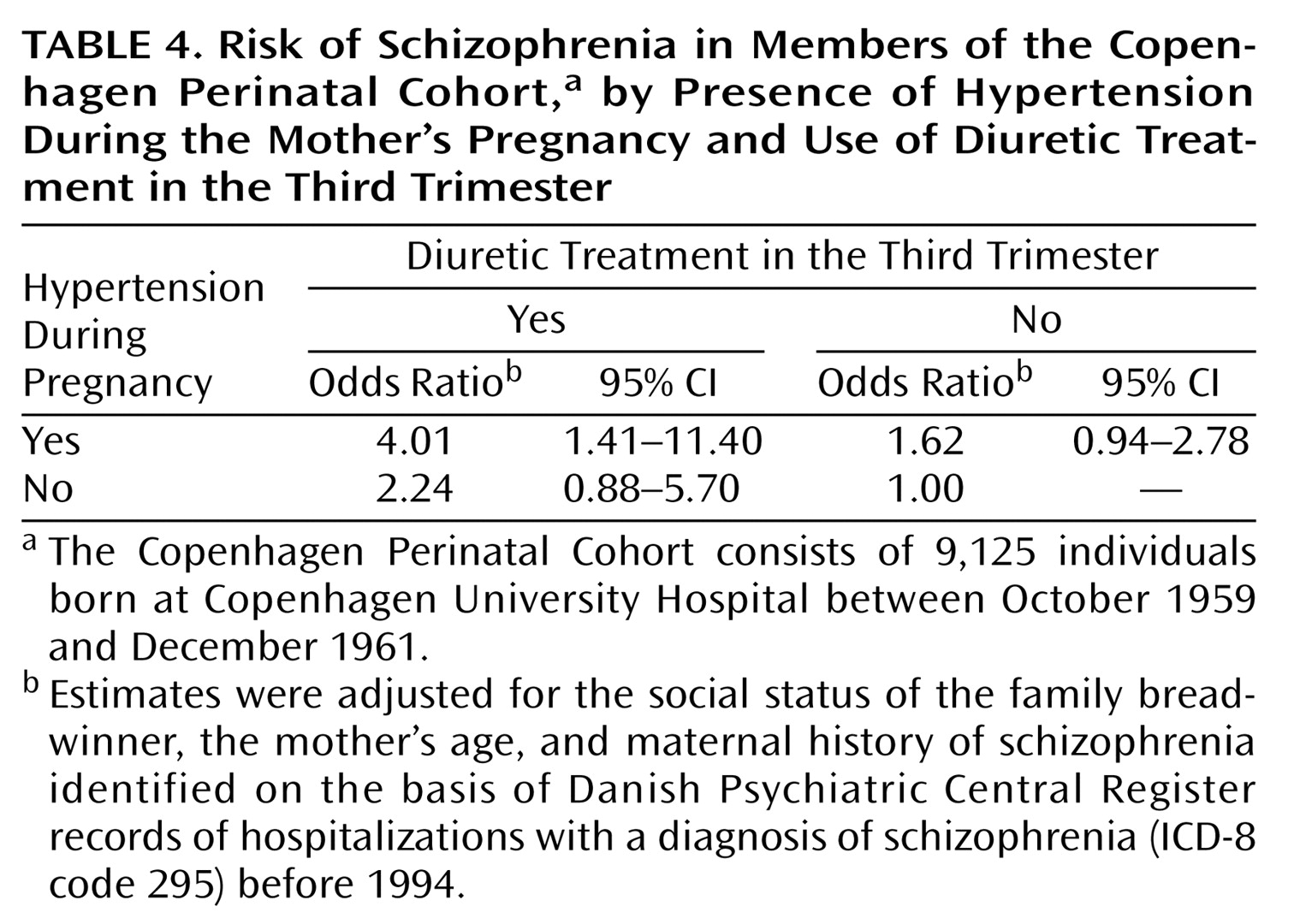
Table 4, diuretics were prescribed in the third trimester for conditions other than hypertension. The reference category in this analysis was pregnancies that were unexposed to both diuretics in the third trimester and hypertension. The presence of maternal hypertension combined with the absence of diuretic treatment was not significantly associated with increased risk of schizophrenia. In the absence of maternal hypertension, prenatal exposure to diuretics was not a significant predictor of the outcome. Including the indexes of pregnancy and delivery complications as covariates did not materially alter the trends showed in
Table 4. Thus, the combination of hypertension and diuretic treatment in the third trimester remained a significant predictor, with about the same predictive capacity in this analysis as in the model without the covariates of pregnancy and delivery complications. The table shows that the combination of maternal hypertension and third-trimester exposure to diuretics was significantly associated with schizophrenia (odds ratio=4.01 [95% CI=1.41–11.40]). The combination of maternal hypertension and third-trimester exposure to diuretics was not significantly associated with other categories of psychotic disorder (ICD-8 codes 296, 297, and 298). Earlier research demonstrated a 2.5-fold elevated relative risk of schizophrenia in offspring for preeclampsia
(10). In this study, however, we did not find a significant association between preeclampsia and increased risk of schizophrenia (
Table 1). A restriction of the cohort by excluding all cases (N=304) with classifications of either mild or severe preeclampsia did not substantially dilute the predictive effect of the combination of maternal hypertension and diuretics presented in
Table 4.
To address the question of differential effects of various diuretic substances, we analyzed more detailed information on prescribed drugs in pregnancy for a subsample of 1,766 individuals from the Copenhagen Perinatal Cohort
(20). In a preliminary analysis with limited statistical power (data not shown), we did not find indications of significant differential effects of various diuretics (e.g., thiazides, loop diuretics, carbon anhydrase inhibitors, or “unknown diuretic cures”) in relation to the outcome.
Discussion
We found an increase in the risk of schizophrenia in offspring prenatally exposed to diuretics during the third trimester and a statistically significant fourfold increase in the risk of schizophrenia in offspring of hypertensive mothers treated with diuretics in the third trimester. The increase in the risk of schizophrenia was statistically significant when the analysis included additional confounders and other risk factors for schizophrenia.
The psychiatric diagnoses in the present study were clinical ICD-8 diagnoses. However, in Denmark, the ICD-8 diagnosis of schizophrenia was used conservatively, and previous studies have shown that almost all patients with a diagnosis of schizophrenia meet the DSM-III-R criteria for schizophrenia
(21). The rather narrow concept of schizophrenia according to ICD-8 is likely to have led to diagnostic caution rather than overinclusiveness. Had we extended the period of follow-up beyond 1994, some late-onset cases of schizophrenia might have been identified, and it is also possible that some cases of paranoid psychoses or reactive psychosis may have been reclassified as cases of schizophrenia after 1994. Prenatal exposure to diuretics or maternal hypertension was, however, not associated with any of these diagnostic categories.
From a methodological viewpoint, our prospective study is well suited to examining putative associations between prenatal exposure to medication and adverse outcomes many years later
(17,
18). The taxonomy and definitions used to diagnose hypertensive disease in pregnancy have evolved during the last 40 years, and it is possible that the term “hypertension” in our data set might refer to conditions that would now be labeled differently (i.e., gestational hypertension or chronic hypertension). It is also possible that the threshold for the diagnosis of preeclampsia may have been higher 40 years ago, and the study lacked statistical power to examine the predictive capacity of this variable. Exposure to drug treatment during pregnancy was recorded a few days after birth, and we do not consider misclassification of exposure to diuretics or other drugs a major problem. Prenatal exposure to diuretics in the second trimester was more rare than exposure in the third trimester, and it is possible that the present study was not large enough to detect an effect of second-trimester exposure in relation to adult outcome.
A methodological advantage was that data on many variables were collected during pregnancy or immediately after the birth, thus minimizing the risk of recall bias. Another advantage was that we were able to control the confounding effect of the social status of the family breadwinner in our analysis. Social status was slightly higher in diuretic-treated hypertensive women than in women without these two factors, and, moreover, lower social status correlated with increased risk of schizophrenia. A minor limitation was that cases of late-onset schizophrenia (after age 34–35 years) were not captured, as follow-up was limited to the period up to 1994.
To establish a drug-associated risk of a specific adult outcome in a nonexperimental setting requires comparability of the exposed group and the nonexposed group. Although we sought to control for relevant confounders, the decision to prescribe diuretics might have been influenced by factors that were not captured by the variables in the database. The indexes of pregnancy and delivery complications may be related to the severity of hypertension, but they are not direct measures of the severity of hypertensive disease in pregnancy. Differences in the severity of maternal hypertension might explain the apparent effects of diuretics if the mothers with the most severe hypertension were systematically more likely to be prescribed diuretics in the third trimester than those with less severe hypertension. Since we cannot rule out that such a prescription pattern may have existed, and since the study was not large enough to establish a specific drug-associated risk, we believe that the current findings should be interpreted with caution.