Cognitive deficits are prominent in many patients with schizophrenia and may predict their social outcome
(1,
2). Neuroimaging studies have been able to link some of these cognitive impairments in schizophrenia to specific neural circuits
(3). We decided to employ the Multi-Source Interference Task
(4), a functional neuroimaging paradigm specifically designed to reliably activate the dorsal anterior cingulate cortex, to examine dorsal anterior cingulate cortex function during cognitive interference in schizophrenia.
The anterior cingulate cortex as a whole provides an interface for motor control, drive, and cognition
(5,
6) and has therefore attracted great interest in the study of schizophrenia
(7). Of importance is that it has been recognized that the anterior cingulate cortex encompasses functional subdivisions that subserve cognitive and emotional processes
(5,
8–10). These anterior cingulate cortex subdivisions are based on distinct cytoarchitectural and connectivity profiles: the dorsal areas 24c′/32′ (
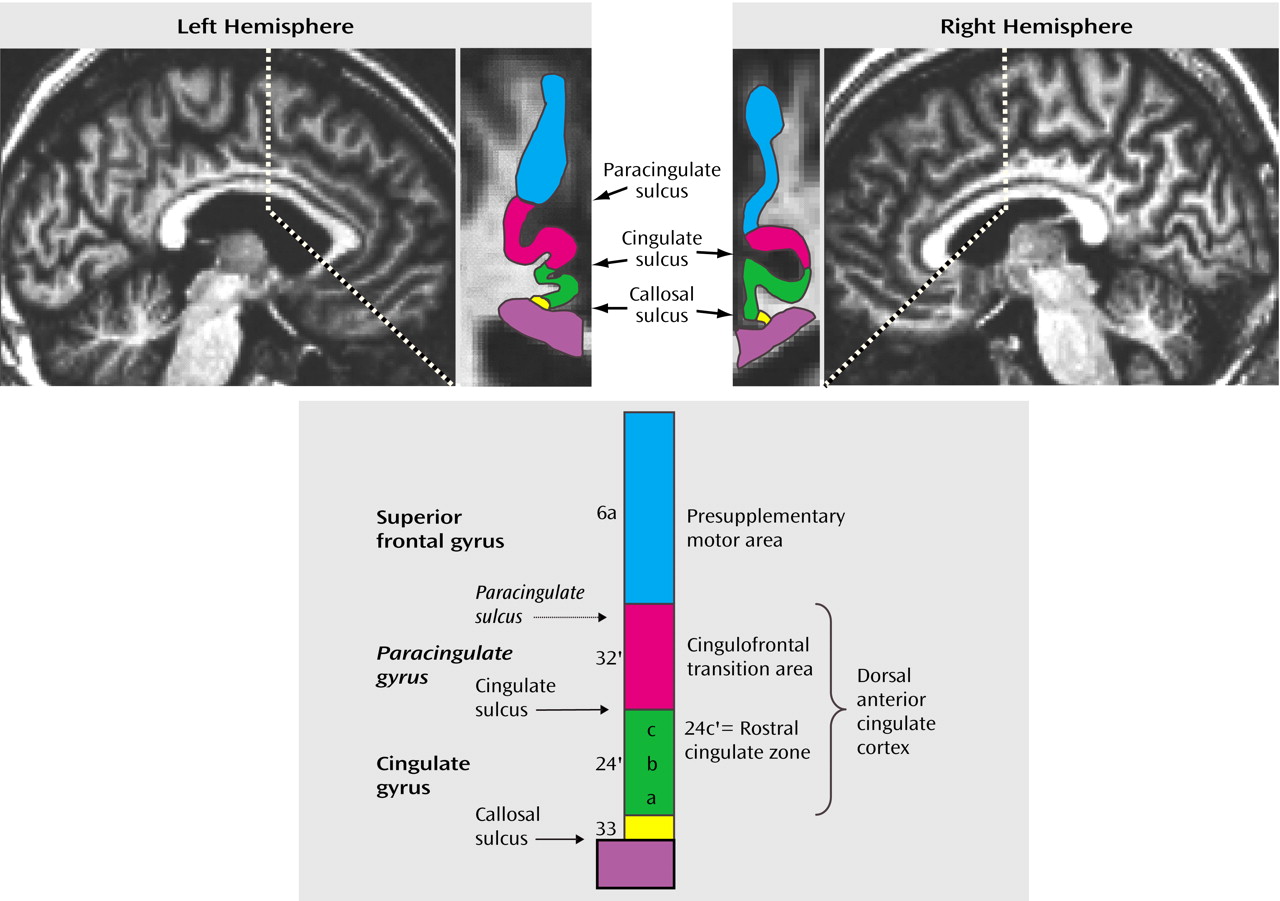
Figure 1) are closely linked with cognitive-motor functions
(8,
11,
12), while the rostral areas 24a-c/32 are associated with limbic functions
(8–
12). Immediately dorsal to areas 24′/32′ is the presupplementary motor area, which is equivalent to area 6a and is connected with motor modules in the dorsolateral prefrontal cortex
(13).
Neuroimaging studies have, in lieu of direct information about such subdivisions for each individual, inferred the location of the anterior cingulate cortex subdivisions from the sulcal/gyral pattern of the medial wall in the human brain. The dorsal anterior cingulate cortex encompasses the sections of the anterior cingulate cortex directly dorsal to the corpus callosum (including the anterior cingulate gyrus and, if present, the paracingulate gyrus), whereas the presupplementary motor area is equivalent to the medial aspect of the superior frontal gyrus (
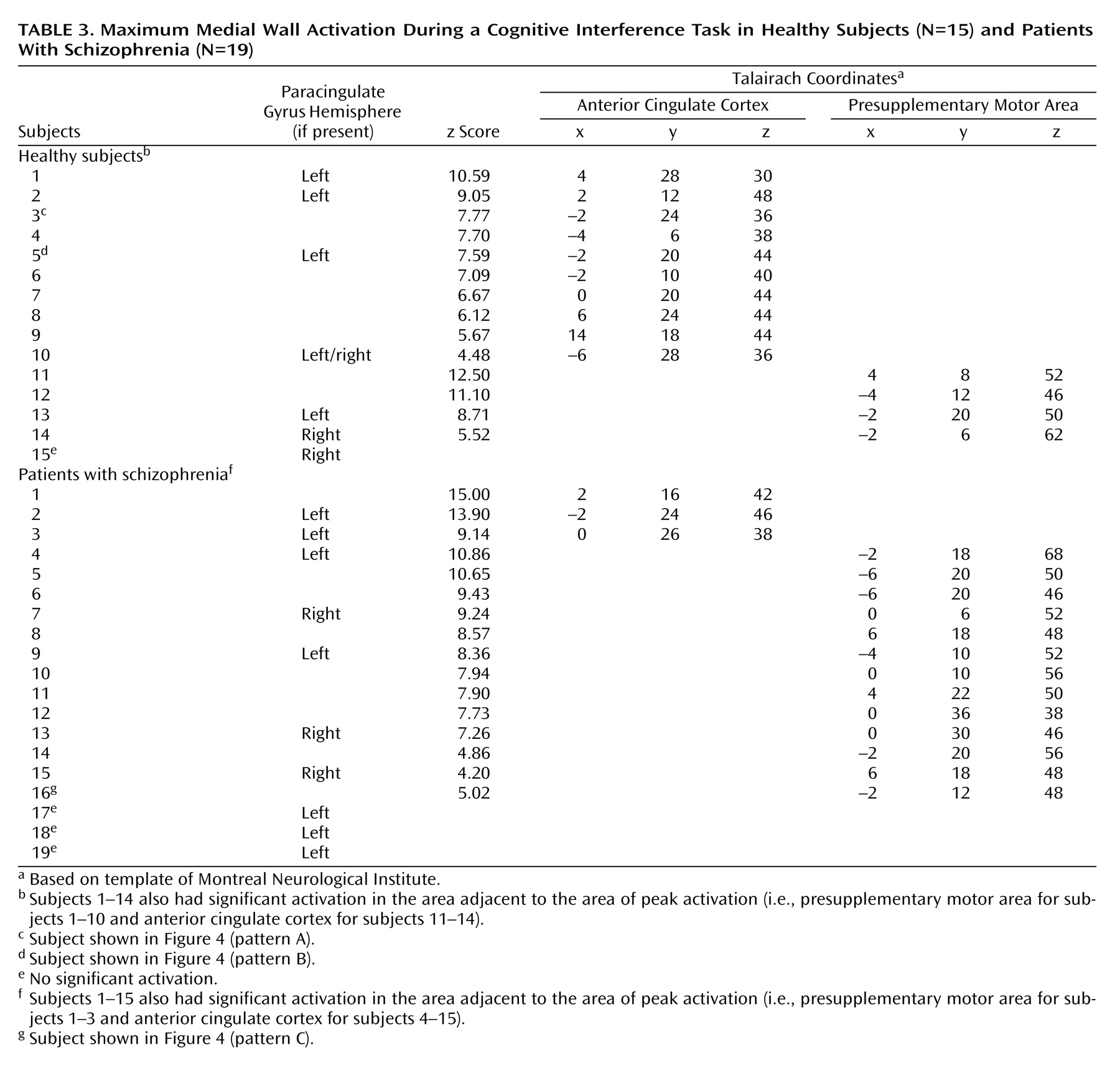
Figure 1). The sulcal pattern of the medial wall, however, is highly variable: a paracingulate gyrus is found in only 30%–50% of healthy individuals
(9,
14–16), occurs more often in the left hemisphere (probably related to the left hemisphere dominance for speech, in which the paracingulate gyrus is involved)
(6), and may be less prevalent in disease states such as schizophrenia
(17). Considering the structural heterogeneity and the distinct functional roles of medial wall regions in the human brain, we decided to study dorsal anterior cingulate cortex function in schizophrenia by using the Multi-Source Interference Task
(4) in conjunction with high-resolution structural magnetic resonance imaging (MRI).
Method
Subjects
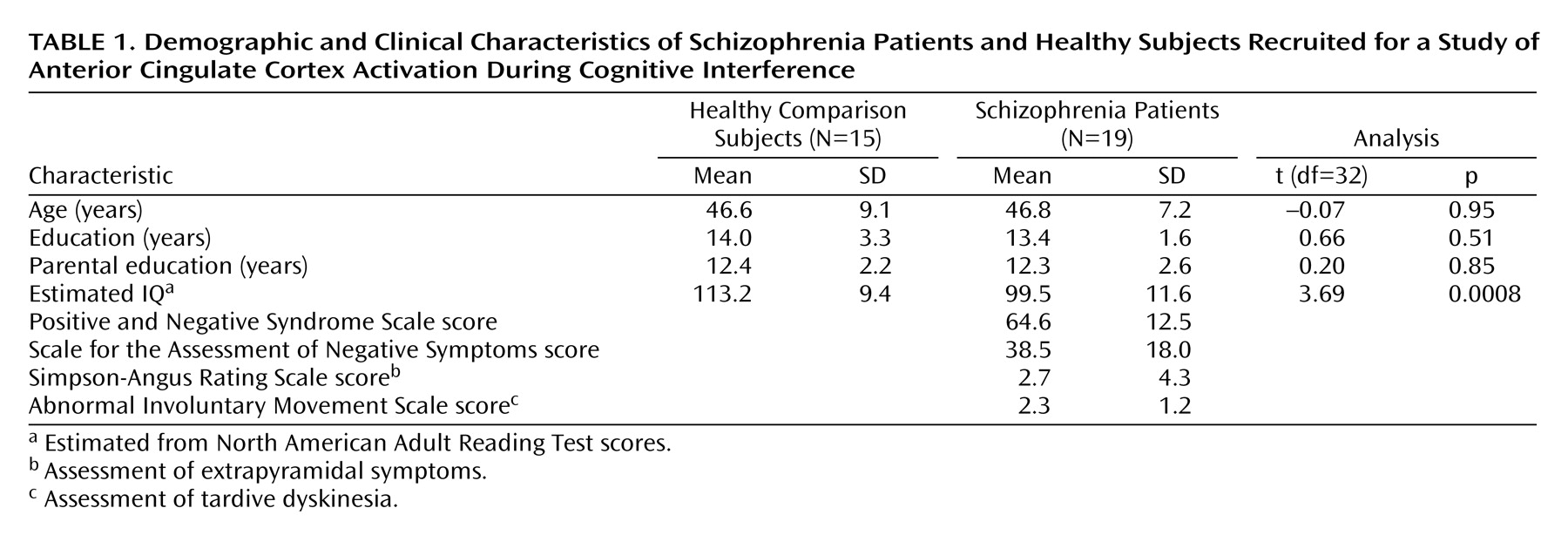
After approval of the study protocol by the institutional review boards of the Massachusetts General Hospital and the Massachusetts Department of Mental Health, we obtained written informed consent from 18 healthy subjects recruited from the community and 25 schizophrenia subjects recruited from an outpatient clinic in Boston. Three schizophrenia subjects were excluded from the neuroimaging experiment for medical reasons (claustrophobia, vision impairment, metal in eye). In addition, one healthy subject and one schizophrenia subject were excluded because their performance accuracy during an offline trial of the experiment was less than 85% (71% and 31%, respectively). Data sets of two healthy and two schizophrenia subjects were incomplete or lost because of technical failure. This resulted in a final study group size of 15 healthy subjects and 19 schizophrenia patients.
No subject had a history of major medical or neurological illness, and no healthy subject was found to have a history of psychiatric illness according to the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)
(18). All patients were chronic, stable outpatients with a history of schizophrenia, as established by the SCID
(18). The two groups were matched in terms of gender, age, and parental education (
Table 1).
Task Description
The Multi-Source Interference Task has been validated and full task details appear in Bush et al.
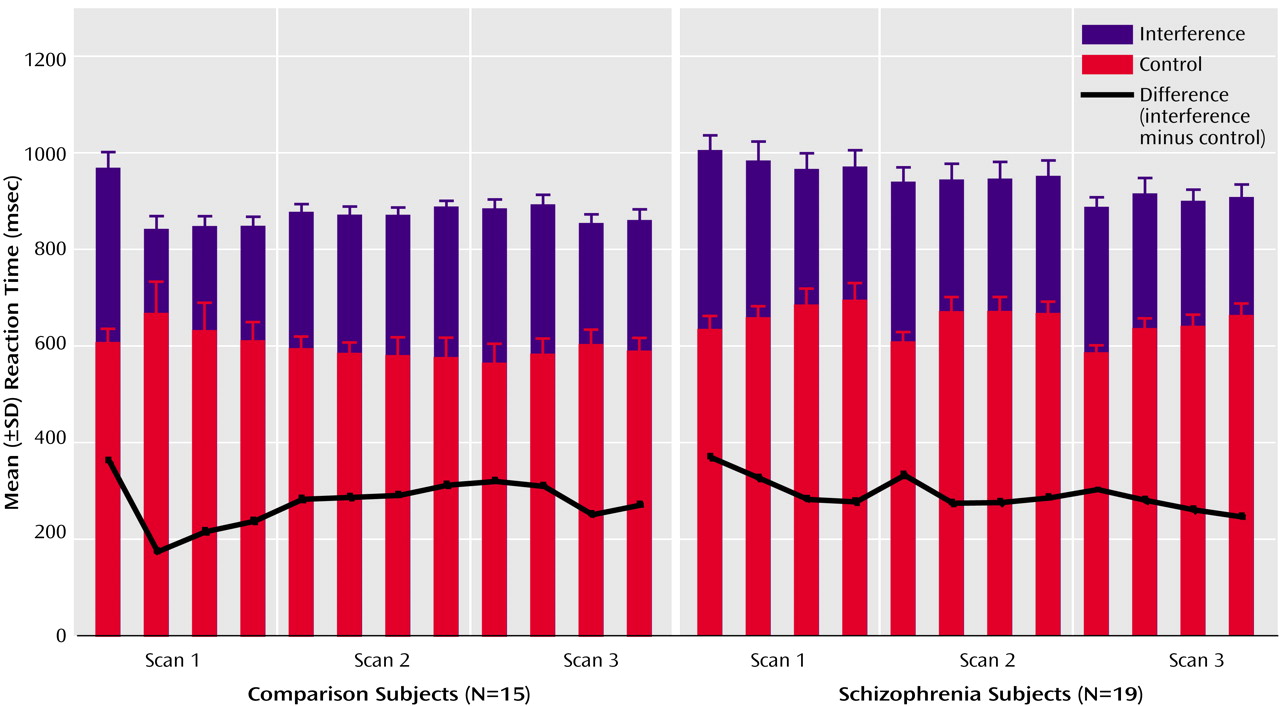
(4). Subjects viewed a set of three items (the numbers 1, 2, or 3 or a lowercase “x”) on a computer screen in front of them and were instructed to indicate the identity (not the position) of the number that was different from the other two items by button press. In control task trials (C), the target was the only large number (distractor cues were lowercase x’s), and the number would always match its position on the keypad (i.e., 1xx, x2x, and xx3). In contrast, during interference trials (I), all three stimuli were numbers and the target could be large or small and would never match its position. Subjects were informed that scans would begin and end with fixation (F) on a white dot for 30 seconds, between which they would see eight alternating blocks of control and interference trials, lasting 42 seconds each. For all trials, subjects were instructed to answer as quickly as possible but to make sure that they gave the correct answer.
After instructions were reviewed, subjects completed a practice of the task (192 trials, presented as four blocks of 24 control trials alternating with four blocks of 24 interference trials). During the neuroimaging experiment the subjects completed three scans (with 192 trials each, presented as eight alternating blocks of control and interference trials, for a total of 576 trials), which were separated by two pauses of 5 minutes each. The order of presentation was fixed within scans (i.e., it was always FCICICICIF).
Functional and Structural MRI Procedures
All subjects were scanned in a Siemens 1.5-Tesla Sonata high-speed echo-planar imaging device (Munich, Germany). Stimuli were generated via MacStim 2.2.1 (West Melbourne, Australia) on a Macintosh Powerbook (Cupertino, Calif.), projected onto a screen, and viewed by the subjects via a tilted mirror placed in front of their eyes.
High-resolution structural images (1.3×1.0×1.3 mm, magnetization-prepared rapid acquisition with gradient echoes, 128 slices, echo time [TE]=2.978 msec; repetition time [TR]=2532 msec per slice, flip angle=7°) were collected for three-dimensional reconstruction before the functional scanning session. Each functional series lasted 6 minutes and 36 seconds. We collected 264 functional brain images (TE/TR=40/1500 msec; 15 coronal slices, perpendicular to the anterior commissure/posterior commissure line and extending anterior from a point approximately at y=–10 mm; 5 mm thickness, no skip; voxel size 3.125×3.125×5 mm; field of view=200 mm; flip angle=90°) to capture 192 trials lasting 1.75 seconds each, bracketed by two blocks of fixation trials, lasting 30 seconds each.
Data Analysis
Reaction time and accuracy data were analyzed by using mixed-model analysis of variance with condition (control, interference) as the within-subject effect and group (healthy subjects, schizophrenia patients) as the between-subject effect.
Structural and functional images were corrected for head motion with SPM 99 (Wellcome Department of Cognitive Neurology, London). For each subject, we coregistered the structural image to the mean functional image. We then transformed the mean functional image into a common reference space (Montreal Neurological Institute [MNI] Talairach brain)
(19) using an EPI template image as the target and applied the resulting transformation parameters to all functional images and the structural image.
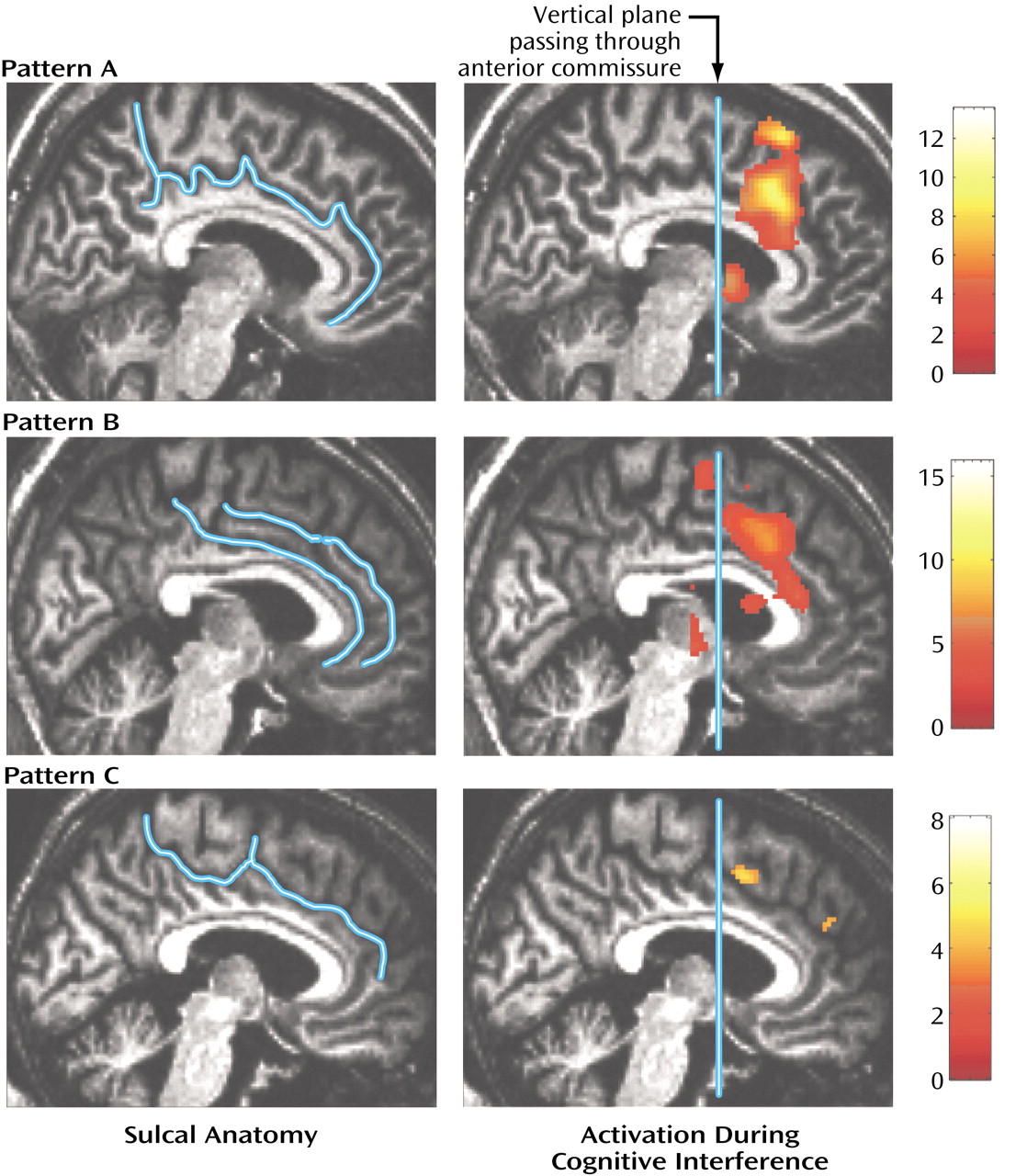
We used an orthogonal viewer of coronal, sagittal, and horizontal sections to classify the sulcal pattern in the medial wall of the prefrontal cortex. We employed criteria described previously
(14,
16) and adjusted them to distinguish two sulcal patterns, i.e., absence or presence of a paracingulate gyrus.
Functional images were smoothed by using an 8-mm full width at half maximum Gaussian filter. For each subject, general linear models were created that included the effects of condition (control and interference) and scan
(1–
3) to explain the variance of blood-oxygen-level-dependent (BOLD) signal change at each voxel. We tested for the effect of condition (i.e., greater BOLD signal during interference compared with control blocks) across the three experimental sessions.
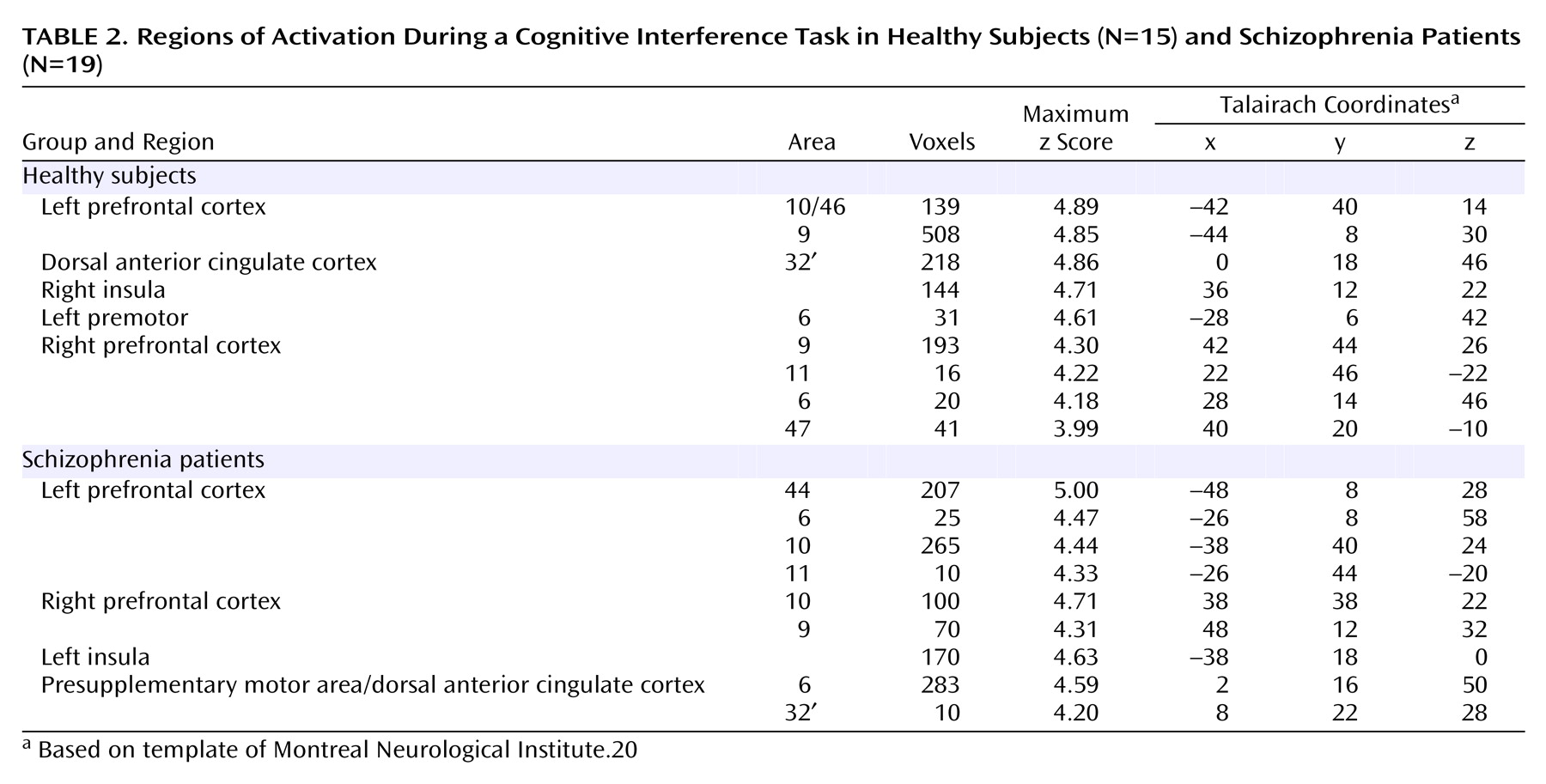
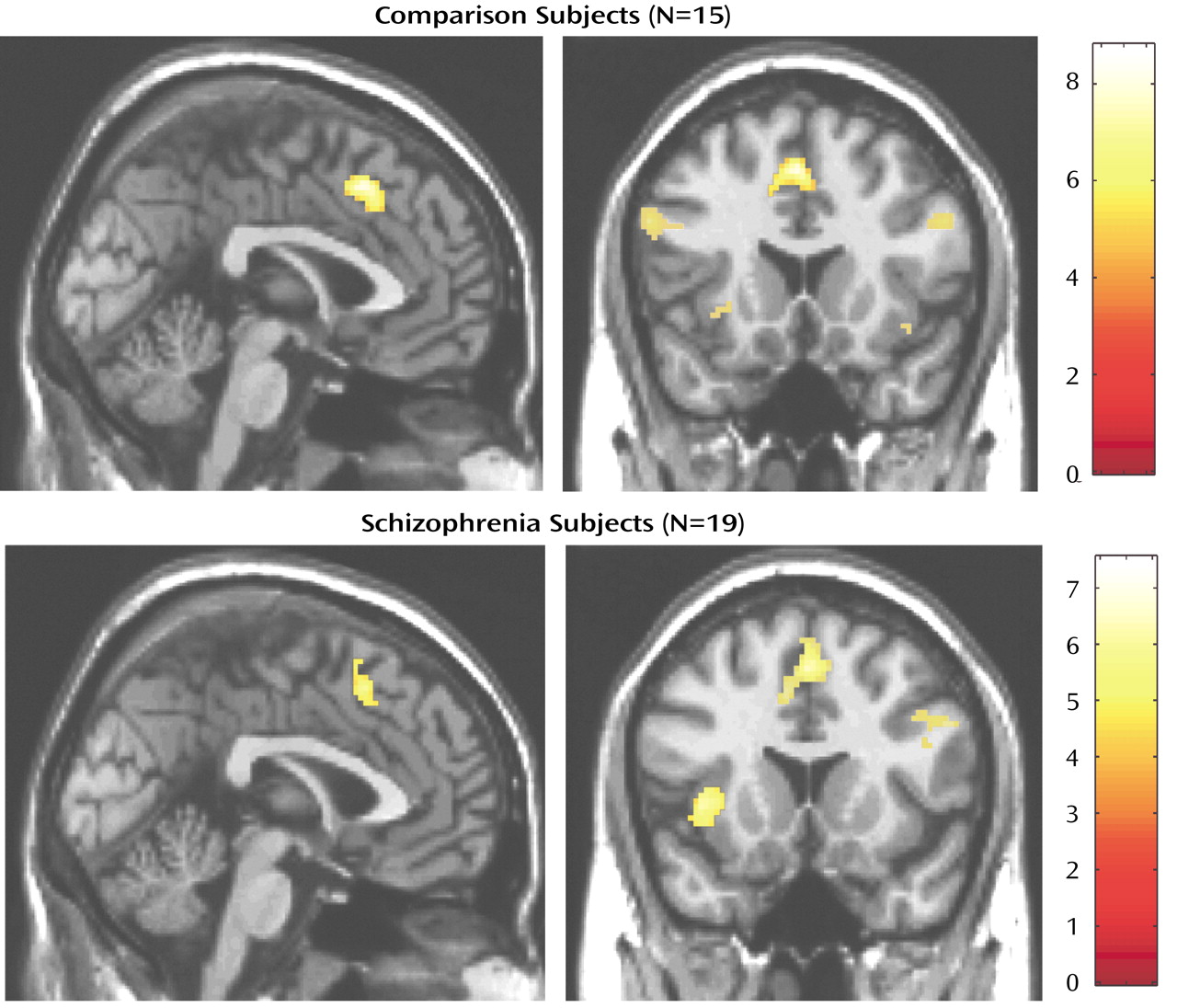
Using these contrast images we tested for differences between healthy and schizophrenia subjects at two levels. First, to test for an overall effect of group we pooled all individual contrast images (thresholded at p<0.05, uncorrected for multiple comparisons) into a one-sample t test (for within-group effects) and a two-sample t test (for between-group effects), using a stringent significance threshold of p<0.0001.
Second, to investigate medial wall activation in each individual subject we displayed contrast images on the coregistered structural images, using a significance threshold of p<0.0001 as a correction for multiple comparisons within the a priori-defined medial wall region of interest
(20). This allowed us to test the hypothesis that schizophrenia is associated with abnormal medial wall activation during cognitive interference in two respects, i.e., cluster extent and location of maximal excursion.
Discussion
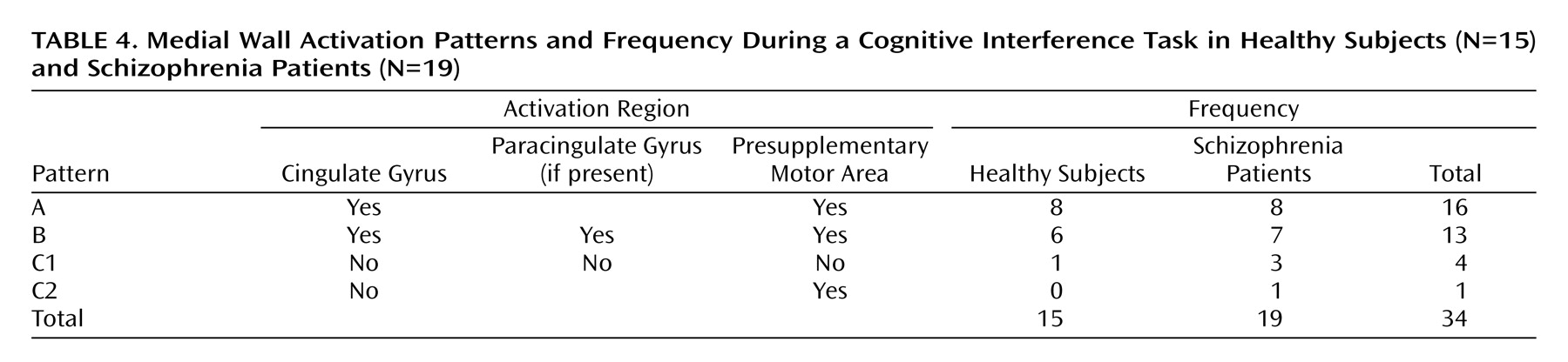
We have demonstrated that cognitive interference, as induced by the Multi-Source Interference Task and analyzed in individual subjects, produces robust medial wall activation in healthy subjects and in most subjects with schizophrenia. Group analysis showed that the overall activation pattern of the schizophrenia group was more dispersed and shifted dorsally, but the mean image did not differ significantly from the normal pattern. Detailed analysis of the individual data, however, revealed that the majority of schizophrenia subjects displayed their maximum medial wall activation outside of the dorsal anterior cingulate cortex and that a subset of schizophrenia subjects failed to activate the dorsal anterior cingulate cortex during cognitive interference.
Our finding of robust dorsal anterior cingulate cortex activation during cognitive interference in healthy subjects is consistent with a previous study that used the Multi-Source Interference Task
(4). Here we identified two normal patterns of medial wall activation on the basis of the presence or absence of a paracingulate gyrus. Most subjects revealed activation of the posterior sector of the rostral cingulate zone in the depth of the cingulate sulcus, i.e., Brodmann’s area 24c′, a region implicated in the control of motor behavior
(13,
21).
The Stroop-like cognitive interference induced by the Multi-Source Interference Task relies on the combination of effects, including the Simon effect (position of target cue information does not correspond to its position on button press) and the Eriksen flanker effect (the target cue is flanked by incongruent distractor cues)
(4). Combined with other factors known to activate the dorsal anterior cingulate cortex (e.g., novelty, errors)
(20,
22,
23), this leads to a more pronounced reaction time prolongation compared with classical Stroop tasks
(4). It is likely that such a combination of interference effects results in more robust medial wall activation
(5). Further studies are needed to understand how the combination of the different Multi-Source Interference Task components contributed to the medial wall activation.
Abnormalities of the anterior cingulate cortex in schizophrenia have been reported in studies of neuronal cell density
(7), sulcal/gyral pattern
(17), spectroscopic analysis of
N-acetylaspartate
(24,
25), modulation of blood flow in response to pharmacological challenge
(26), and functional activation during task performance
(27–
31). Taken together, these findings have been interpreted as evidence for anterior cingulate cortex pathology in schizophrenia
(7). However, it has become clear that the term anterior cingulate cortex refers to structurally and functionally distinct areas in the medial wall of the prefrontal cortex. One distinction especially relevant for schizophrenia is the rostral/dorsal parcellation into areas 24/32 (which receive strong projections from the amygdala) and 24′/32′ (which are connected with prefrontal, parietal, and motor areas)
(12,
32). This pattern has led some investigators to propose a combined role of amygdala and anterior cingulate cortex areas 24/32 in the production of affective behaviors, whereas areas 24c′ and 32′ have been linked to attention and motor function
(8,
33). Neuropathological studies of schizophrenia subjects have reported cell loss and abnormal neuropil in area 24, which has been interpreted as evidence for abnormalities of amygdala afferents into the anterior cingulate cortex
(7). It is not known whether schizophrenia is also associated with abnormal cellular architecture of the anterior cingulate cortex region studied here, i.e., posterior areas 24′/32′.
Initial studies employing the Stroop task were interpreted as evidence for an impaired ability to process competing stimulus features in schizophrenia
(34). However, a thorough review revealed that this inference was often based on an incomplete or incorrect analysis of the reaction time and accuracy data, and that differences in experimental design (i.e., block or single-trial design) could explain some of the discrepancies between the various studies
(34). Our finding of similar interference effects on reaction time in schizophrenia is in line with previous studies
(34).
Previous positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies of blocked-design, classical Stroop tasks have reported decreased activation in both rostral and dorsal subdivisions of the medial wall during cognitive interference blocks in schizophrenia
(28–
31). A recent fMRI study of error-related activity in the anterior cingulate cortex revealed further compelling evidence for abnormal anterior cingulate cortex activation (significant group-by-accuracy-by-scan interaction at coordinates 2, 21, 36) in schizophrenia
(35). The recent study by Yücel et al.
(31) is particularly relevant for our discussion, since the authors reported decreased cingulate gyrus/paracingulate gyrus but normal presupplementary motor area activation in five subjects with schizophrenia. Two experimental details could explain why we detected dorsal anterior cingulate cortex activation in the majority of subjects with schizophrenia. First, we used the Multi-Source Interference Task, which most likely resulted in more significant medial wall activation. Second, we studied BOLD signal at 1.5 T, which appears to be more accurate for mapping the individual pattern of medial wall activation. Despite these differences, we agree with Yücel et al. that some patients with schizophrenia lack dorsal anterior cingulate cortex activation and that some show more robust presupplementary motor area activation during cognitive interference.
Brain activation during cognitive interference in schizophrenia deserves further study. The normal reaction time and accuracy during the performance of the Multi-Source Interference Task in the four subjects without significant dorsal anterior cingulate cortex activation indicates that additional brain regions are involved in performing cognitive interference tasks
(4,
6). To study such alternate patterns of brain activation and to elucidate their behavioral consequences we need paradigms, such as the Multi-Source Interference Task, that ensure robust and consistent activation of the region of interest at the level of individual subjects. Our findings are a compelling example of how refined tasks and the analysis of individual data can improve group data analysis, especially in highly variable regions of the human cerebral cortex.
In conclusion, maximum medial wall activation outside of the anterior cingulate cortex during cognitive interference and lack of anterior cingulate cortex activation in a subgroup of schizophrenia subjects provide further evidence for an abnormality of the anterior cingulate cortex in schizophrenia.