Substantial evidence from laboratory studies of animals demonstrates that dopamine release in the ventral striatum underlies the reinforcing properties of nicotine
(1,
2). Microdialysis
(3–
6), lesion
(7), and imaging
(8) studies in rats indicate that nicotine-induced dopamine release is strongest in this region and is more robust than the dopamine release found in associated structures receiving dopaminergic input, such as the dorsal striatum
(3). Several of these studies used nicotine doses that simulated human cigarette smoking
(4,
7,
8), and it has been reported that nicotine-induced dopamine release is comparable in magnitude to that induced by other addictive drugs
(4). A study of nonhuman primates
(9) that used the [
11C]raclopride positron emission tomography (PET) method also found nicotine-induced ventral striatum dopamine release. In addition, one report
(10) described a difference between smokers and nonsmokers in
18F-fluorodopa uptake (a measure of presynaptic dopamine activity) during PET imaging. Smokers had greater tracer uptake in the caudate and putamen than nonsmokers, suggesting greater dopaminergic turnover in smokers. However, to our knowledge, there have been no published reports of smoking-induced ventral striatum dopamine release in humans.
In recent years, the [
11C]raclopride bolus-plus-continuous-infusion PET method has been used increasingly for the (indirect) measurement of dopamine release in response to addictive drugs
(11). [
11C]Raclopride binds with relative specificity to dopamine D
2 (and D
2-like) receptors and has low affinity for D
1 receptors
(11,
12). [
11C]Raclopride binding potential (a measure of D
2 receptor occupancy) has been demonstrated to have an inverse linear relationship with extracellular dopamine concentration, as measured with microdialysis in nonhuman primates
(13). Because synaptic dopamine competes with [
11C]raclopride for D
2 receptor binding, dopamine release is thought to decrease [
11C]raclopride binding potential directly
(11). This method has been used to demonstrate changes in synaptic dopamine concentration in response to cocaine
(14), amphetamine
(15–
17), and methylphenidate
(18) administration in humans, as well as to nicotine
(9,
19), cocaine
(20,
21), and amphetamine
(22,
23) administration in animals. Decreases in [
11C]raclopride binding potential have also been demonstrated in response to nonpharmacological stimuli, such as placebo administration
(24), meditation
(25), and playing a video game
(26). Test-retest reliability of the [
11C]raclopride method is strong, especially for binding potential
(27,
28), the measure of [
11C]raclopride binding used here.
We sought to determine ventral striatum dopamine release in response to cigarette smoking in nicotine-dependent human subjects by using [11C]raclopride PET. We also sought to determine if smoking-induced changes in dopamine concentration were associated with changes in smoking-related clinical states, such as craving and anxiety.
Results
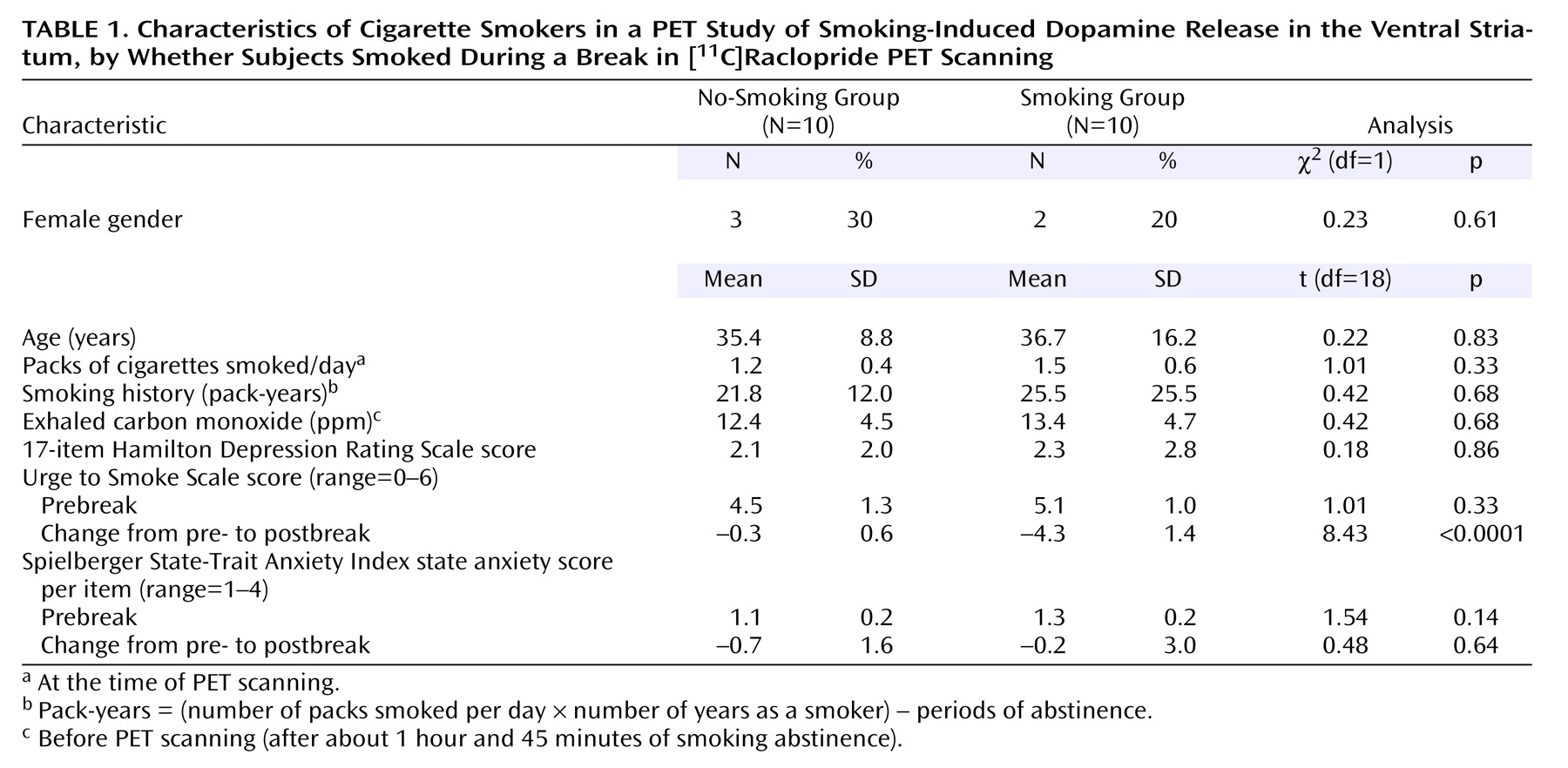
There were no significant differences between the groups at baseline in demographic characteristics, CO levels, or rating scale scores (
Table 1). There were also no significant prebreak between-group differences in binding potential for any of the regions of interest (Student’s t tests, two-tailed, range of p values=0.14 to 0.89). As expected, from pre- to postbreak, the group that smoked had a significantly greater reduction in craving (mean Urge to Smoke Scale score) than the group that did not smoke (Student’s t test, two-tailed, p<0.0001). No difference was found between groups in pre- to postbreak change in state anxiety (as measured by the State-Trait Anxiety Inventory) (Student’s t test, two-tailed, p=0.64).
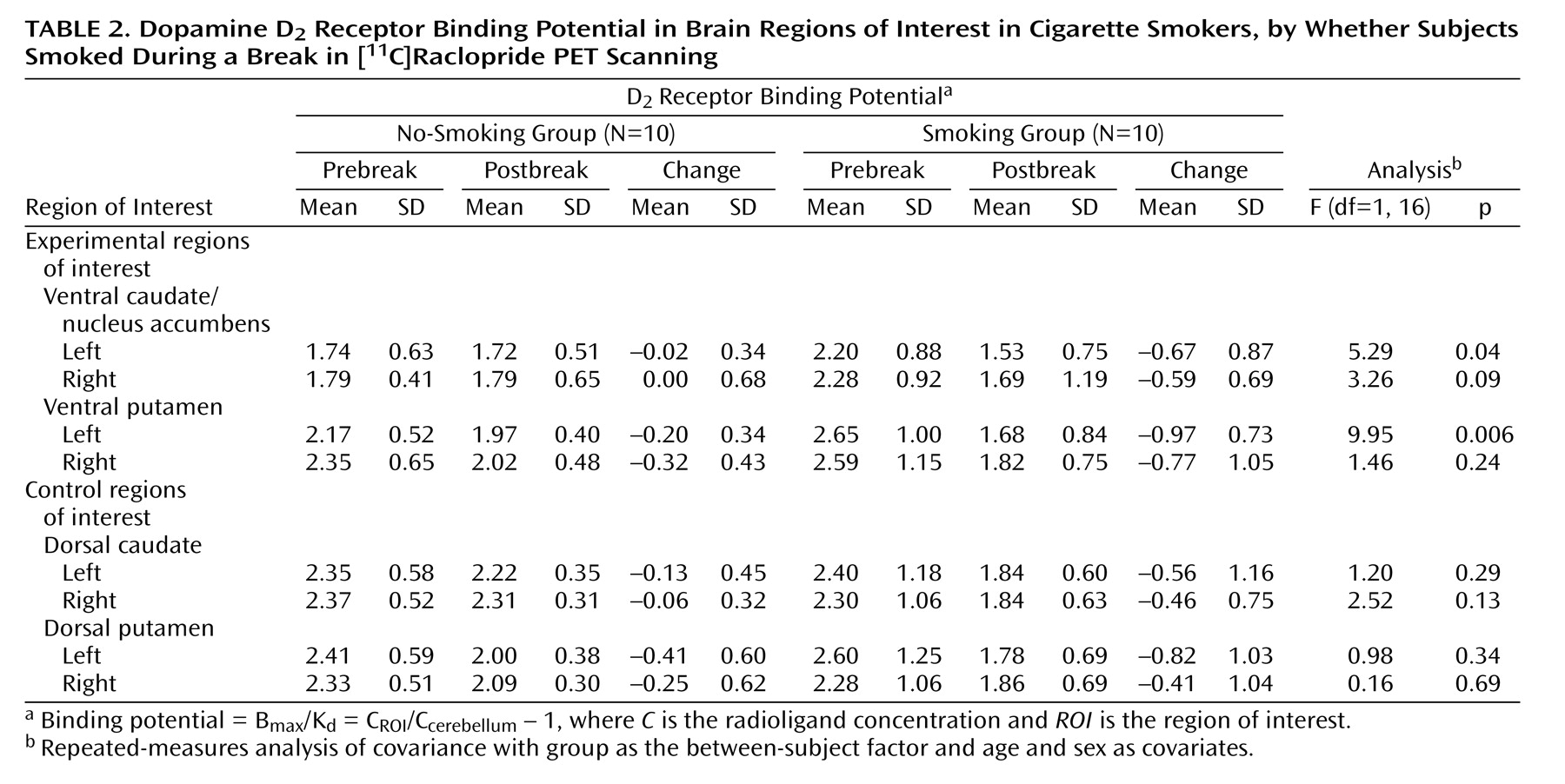
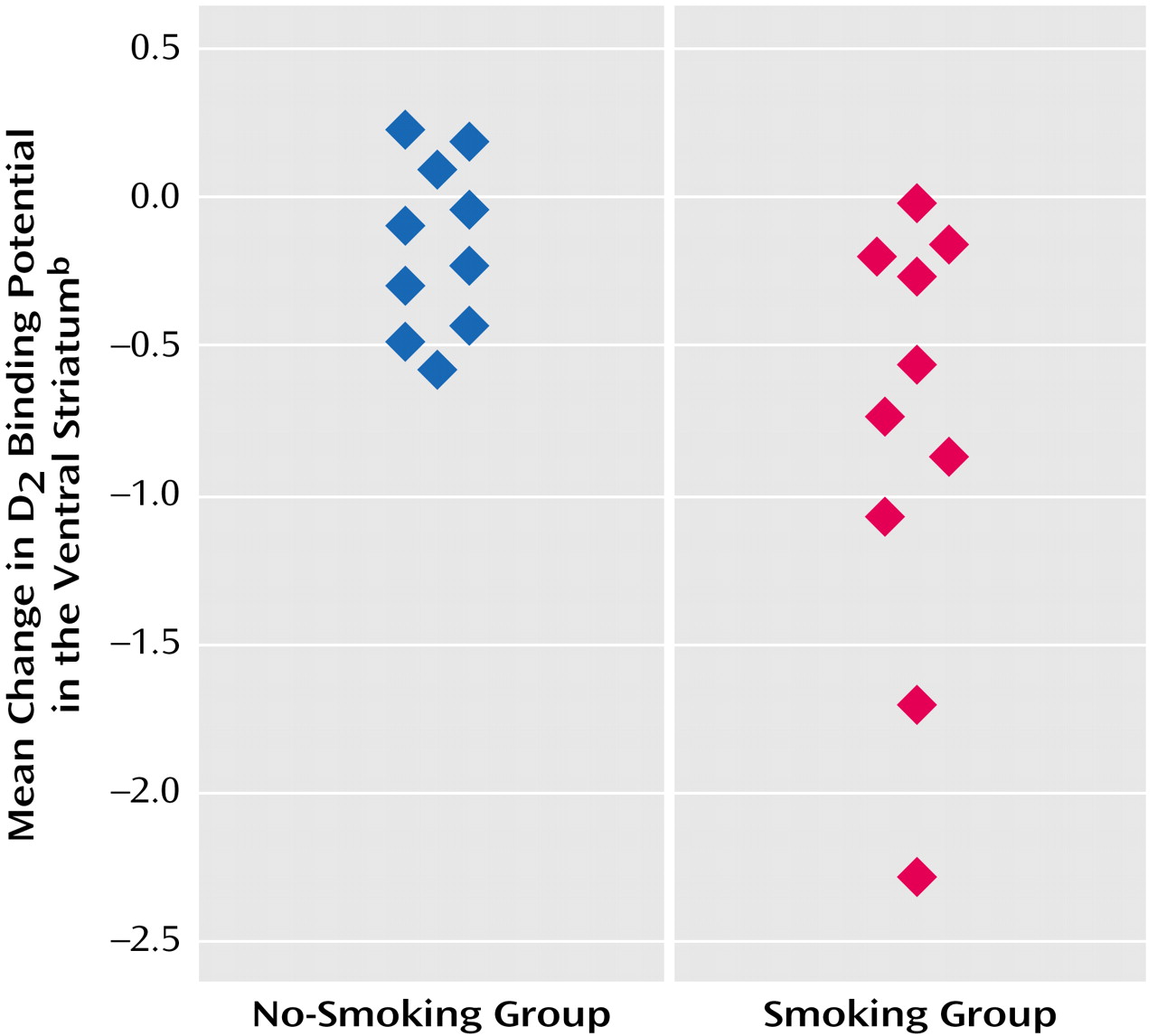
In the overall repeated-measures MANCOVA, a significant between-group effect was found (F=7.6, df=1, 16, p<0.02), indicating an overall group difference in change in [
11C]raclopride binding potential in ventral striatum regions between those who did and did not smoke (
Figure 2). Follow-up repeated-measures ANCOVAs for specific regions demonstrated that the group that smoked had significantly greater reductions in binding potential in the left ventral caudate/nucleus accumbens (F=5.3, df=1, 16, p=0.04) and left ventral putamen (F=9.9, df=1, 16, p=0.006) than the group that did not smoke (
Table 2). The difference in the reduction in binding potential in the right ventral caudate/nucleus accumbens did not reach significance (F=3.3, df=1, 16, p=0.09). The right ventral putamen and control regions of interest did not have significant between-group differences in binding potential change. Percent changes in the experimental regions of interest for those who did not smoke versus those who did smoke were –1.1% versus –30.5% for the left ventral caudate/nucleus accumbens, –9.2% versus –36.6% for the left ventral putamen, 0.0% versus –25.9% for the right ventral caudate/nucleus accumbens, and –13.6% versus –29.7% for the right ventral putamen. All regions of interest had greater mean reductions in binding potential from pre- to postbreak in the group that smoked than in the group that did not smoke (
Table 2).
Among the regions in which differences reached significance in the preceding analysis, the change in binding potential in both the left ventral caudate/nucleus accumbens (r=0.49, df=18, p=0.04) and putamen (r=0.65, df=18, p=0.004) was significantly and positively associated with change in the Urge to Smoke Scale score, possibly indicating that dopamine release in these regions is associated with decreased craving.
Discussion
Nicotine-dependent subjects who smoked a cigarette during PET scanning had greater reductions in [11C]raclopride binding potential (an indirect measure of dopamine release) than nicotine-dependent subjects who underwent the same procedure but did not smoke. These group differences were significant in the ventral striatum (left ventral caudate/nucleus accumbens and putamen; the difference for the right ventral caudate/nucleus accumbens did not reach significance) and were numerically present in all basal ganglia regions studied (including both ventral and dorsal regions). These results provide evidence for smoking-induced dopamine release in humans. Furthermore, reductions in binding potential in the left ventral caudate/nucleus accumbens and putamen were significantly associated with reductions in self-reports of craving, possibly indicating an association between dopamine release and craving reduction.
The magnitudes of smoking-induced decreases in ventral striatal [
11C]raclopride binding potential (–25.9% to –36.6%) here were similar to those found in human (and nonhuman primate) studies that used the [
11C]raclopride PET method paired with administration of amphetamine (–15% to –42%) (15–17, 22, 23), cocaine (–29%)
(20), or methylphenidate (–32%)
(20). Changes here were also greater than those observed in studies that used similar methods paired with nonpharmacological stimuli (–7.9% to –21.2%)
(24–
26). Microdialysis studies in animals have demonstrated that doses of nicotine that emulate human smoking result in roughly a doubling (on average) of extracellular dopamine concentration (range=26% to 600%, depending on dose) in the nucleus accumbens
(3–
6,
8). Furthermore, one study indicated that dopamine concentrations increase more with cocaine (330%)
(3) or amphetamine (550%)
(3) than with nicotine, while another
(4) indicated similarities between nicotine and other addictive drugs.
There are several potential explanations for the similarity between the magnitude of the current findings and those of studies of other addictive drugs. In [
11C]raclopride PET studies of other drugs, nonaddicted subjects have most often been studied. In contrast, our study included nicotine-dependent smokers and was designed to elicit the greatest possible change in ventral striatum binding potential by examining change in this measure in nicotine-dependent subjects at a time point (after 3 hours of abstinence) when craving for cigarettes has been reported to be at its peak
(34) and then having them smoke. Indeed, subjects in this study had a mean craving score of about 80% of the maximum score on the Urge to Smoke Scale (
Table 1) at the 3-hour time point before the break in scanning. This design feature may have maximized the changes in binding potential so that they were comparable to those seen in prior studies with potent dopamine-releasing drugs (e.g., amphetamine, cocaine, or methylphenidate). An alternative explanation is that there is an upper limit to possible displacement of [
11C]raclopride binding potential detectable with PET and that all dopamine-releasing drugs reach this limit of displacement. A third possibility is that in humans smoking produces changes in dopamine concentration that are comparable to those seen with other drugs of dependence, regardless of the subjects being studied.
The correlation found here between craving and left ventral caudate/nucleus accumbens (and putamen) binding potential is consistent with the theory that increases in ventral striatum dopamine concentration are associated with craving reduction and satiety. This relationship is supported by research with animal models of nicotine dependence
(43), other drug dependencies
(44,
45), food
(46,
47), and sex
(48,
49). The current findings are also in agreement with the prior report of euphoria ratings correlating inversely with [
11C]raclopride binding potential in the ventral striatum
(17) (assuming that craving and euphoria are opposing states).
Study findings also support the use of medications that modulate the dopamine system for the treatment of nicotine dependence. Bupropion hydrochloride (HCl) is widely used in the treatment of nicotine dependence and is known to block presynaptic reuptake of dopamine
(50,
51), although this effect has been reported to be somewhat weak
(52). Bupropion HCl has also been shown to increase synaptic ventral striatum dopamine concentrations with both acute
(53) and chronic
(54) administration, although at least one study found greater effects on nondopaminergic systems with bupropion HCl
(55). In addition, other medications that enhance dopamine neurotransmission, such as the dopamine agonist bromocriptine
(56) and the monoamine oxidase-B inhibitor
l-deprenyl
(57) decrease the number of cigarettes smoked in nicotine-dependent subjects and reduce associated withdrawal symptoms. Dopamine receptor antagonists (including haloperidol
[58] and others
[43]) have also been shown to acutely diminish smoking behavior, although one study reported the opposite effect with haloperidol
(59). Thus, change in dopaminergic tone (either through enhancement of dopamine neurotransmission, acute stimulation, or acute blockade) appears to be effective in reducing smoking behavior in nicotine-dependent subjects.
Results of the study should be interpreted in the context of several limitations. First, subjects were removed from the scanner and repositioned after smoking, which could potentially lead to differences in region placement on pre- and postbreak scans because of imperfect repositioning. Although efforts were made to reposition the subjects precisely (by using a laser light from the scanner and markings on the subject’s head) and to draw regions by using the same criteria on pre- and postbreak scans, the possibility remains that region placement was different for the two scans. For the present study, removing subjects from the scanner to smoke was done in a deliberate attempt to emulate naturalistic smoking (based on the hypothesis that this method would lead to the greatest change in dopamine concentration). A second potential confound is that smoking might alter [
11C]raclopride binding potential by effects on blood flow
(60,
61) rather than dopamine release. If global blood flow increased uniformly and radioligand delivery to the striatum and cerebellum increased equally, the [
11C]raclopride binding potential ratio would be expected to decrease in those who smoked. However, in the current group of subjects, cerebellar radioactivity did not increase significantly for either study group (–4% for the smoking group and –2% for the comparison group). Furthermore, [
15O]H
2O PET studies have not demonstrated changes in striatal blood flow in response to nicotine
(19,
62–65). Taken together, these findings indicate that it is unlikely that smoking-related blood flow changes confounded our study results. Third, although subjects were monitored continuously during the break while they were standing in the outdoor area, subtle movements of hands or feet were not systematically examined. Rapid repetitive movements, such as finger tapping
(66) and foot extension/flexion
(67) (but not vigorous exercise
[68]), have been shown to decrease [
11C]raclopride binding potential, although the decreases in binding potential in the past studies were generally smaller than those seen here. A fourth limitation was the modest number of subjects.
To our knowledge, this study is the first to demonstrate dopamine release (indirectly) in response to human cigarette smoking and craving alleviation. The present study supports continued attempts to develop medications that alter dopaminergic tone for the treatment of nicotine dependence.