Following initial reports of pathological fractures in patients suffering from major depressive disorder, several other groups also described low bone mineral density in major depressive disorder and other psychiatric disorders
(1–
3). Bone mineral density was found to be 6%–15% lower in subjects with major depressive disorder than in matched control subjects, and osteoporosis was observed in up to 42% of the patients
(3). Low bone mineral density is associated with a greater risk of bone fractures. The estimated risk increases by a factor of 1.5 to 3 for each reduction of standard deviation in bone mineral density
(4). Since osteoporosis is often a silent disease that might go undiagnosed for a long time, identification of risk factors associated with the development of low bone mineral density may be crucial to identifying groups at risk for osteoporosis and preventing further bone mineral reduction.
A dysregulation of the hypothalamic-pituitary-adrenal (HPA) system, with higher concentrations of serum cortisol in major depressive disorder, has frequently been reported (reviewed in reference
7). Given the reports of osteoporosis in patients who are chronically treated with glucocorticoids or who suffer from Cushing’s syndrome, higher serum cortisol concentrations have been hypothesized to be one mechanism by which depression might induce bone loss
(3). Furthermore, a dysregulation of pro- and antiinflammatory cytokines in major depressive disorder has been reported, including increased serum levels of interleukin-1β, interleukin-2 (IL-2), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and the soluble IL-2 and IL-6 receptors
(8–
14). IL-6 and TNF-α have also been implicated in bone resorption attributable to their activation of osteoclastic cells
(15,
16). Therefore, it seems reasonable to hypothesize a role of cytokines in the pathophysiological process underlying the bone loss in major depressive disorder. However, the exact pathogenic mechanisms of osteoporosis in major depressive disorder have not been sufficiently characterized.
Our main a priori hypothesis was that young women with major depressive disorder already suffer from low bone mineral density. Because of the heterogeneous literature with respect to the type of alterations of bone metabolism in major depressive disorder, we were not able to formulate a directed a priori hypothesis (high versus low bone turnover osteoporosis) with sufficient certainty, but we expected higher bone turnover in the context of elevated concentrations of proinflammatory cytokines.
Approximately 70% of major depressive episodes in young women occur in the context of personality disorders
(17,
18). Among women admitted to the hospital with depressive disorders, the leading associated personality disorders are borderline personality disorder (up to 30% comorbidity) and avoidant personality disorder
(17). We decided to start the exploration of bone density and bone metabolism in young women with depressive disorders in the context of borderline personality disorder and to compare them with women who had borderline personality disorder but no current or lifetime major depressive disorder as well with healthy women.
Method
This study included 38 female patients who were consecutively admitted to our specialized unit for treatment of borderline personality disorder and who met DSM-IV diagnostic criteria for borderline personality disorder. Diagnoses were determined by using German versions of the Structured Clinical Interview for DSM-IV (SCID) and the SCID for Personality Disorders. Sixteen of the 38 patients with borderline personality disorder had no history of major depressive disorder and no current major depressive episode; 22 of the 38 patients were diagnosed as suffering from comorbid major depressive disorder. Ten of these 22 patients had current major depressive episode, and the remaining 12 had a lifetime history of major depressive disorder but no current major depressive episode.
Exclusion criteria for the patients were current or lifetime anorexia nervosa, schizophrenia, oligophrenia, pregnancy, estrogen deficiency, infectious or (auto-) inflammatory disease, and age of 17 years or younger. Twenty healthy normal-weight women of similar age served as the comparison group. Exclusion of psychiatric disorders in the comparison group was also done by using the SCID.
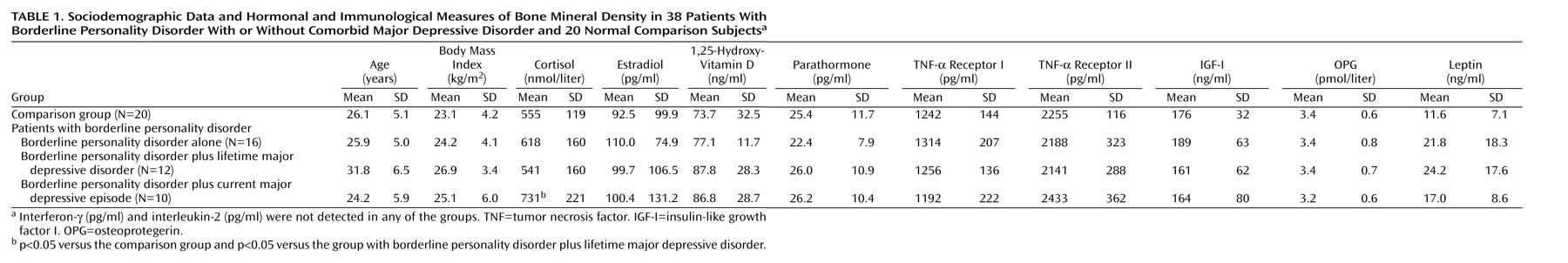
Sociodemographic data for the participating subjects are presented in
Table 1. Eleven of the 38 patients received selective serotonin reuptake inhibitors (three of the 16 patients with borderline personality disorder alone, three of the 12 with borderline personality disorder plus lifetime major depressive disorder, and five of the 10 with borderline personality disorder plus current major depressive episode) for the treatment of bulimia nervosa or the current depressive episode. None of the patients received treatment with mood stabilizers, benzodiazepines, or neuroleptics. The study was approved by the local ethics committee. All patients gave their written informed consent.
Bone mineral density was measured in all patients by means of dual-energy x-ray absorptiometry at the lumbar spine, right femur, left femur, and the forearm of the nondominant hand with a Lunar Prodigy Densitometer equipped with software containing data for an adult reference population (version 2.15.092, Lunar Corp., Madison, Wis.). These normative data were collected from a large sample of healthy subjects enrolled in longitudinal studies of bone density and are specified for age, gender, body mass index, and ethnic group
(19). Staff members involved in the determination of bone density were aware of the patient status of the subject but not of comorbid diagnoses. Bone mineral density was not measured in the healthy comparison group, in compliance with government regulations restricting the use of radiation in healthy subjects. Individual bone mineral density values were expressed as z scores for group comparisons within the patient subgroups (borderline personality disorder plus current major depressive episode; borderline personality disorder plus lifetime major depressive disorder; borderline personality disorder alone), and T scores were used to estimate the fracture risk. Osteopenia was defined in accordance with the World Health Organization guidelines
(20) as a T score ≤–1.
Serum was taken after an overnight fast and stored at –40˚C until analysis. Intact parathormone, 1,25-hydroxy-vitamin D, and laboratory markers of bone turnover (intact osteocalcin and C-terminal telopeptides of type-I collagen, referred to as crosslaps) served as laboratory markers of bone turnover and were determined by using commercial immunoradiometric assay (Nichols Institute Diagnostics, Bad Vilbel, Germany) and enzyme-linked immunosorbent assay (ELISA) (Osteometer Biotech A/S, Herlev, Denmark) kits, respectively. Osteocalcin (a biochemical indicator of bone formation) and crosslaps (a biochemical indicator of bone resorption) were shown to specifically and sensitively reflect alterations in bone metabolism in patients with and without bone diseases and to correlate with bone mass
(21,
22). Cortisol was determined by the radioimmunoassay technique (DPC, Naunheim, Germany). TNF-α receptors I and II, IL-2, interferon-γ (IFN-γ), insulin-like growth factor I, leptin, and osteoprotegerin were determined by using available ELISA kits according to the manufacturer’s instructions (all from R&D Systems, Wiesbaden, Germany). High-sensitivity ELISA kits were used to determine TNF-α and IL-6 (Quantikinine, R&D Systems).
Data were analyzed by using SPSS (version 10.0, SPSS, Inc., Chicago). Analysis of variance (ANOVA) and the Mann-Whitney U test (for z scores of the patient groups) were used to compare study groups. Further analysis was performed with analysis of covariance (ANCOVA), and Pearson’s coefficients of correlation were calculated. The t test was used to compare the z scores of patients with those of the reference population. A p value below 0.05 (two-tailed) was considered significant. All values are given as means and standard deviations when appropriate.
Results
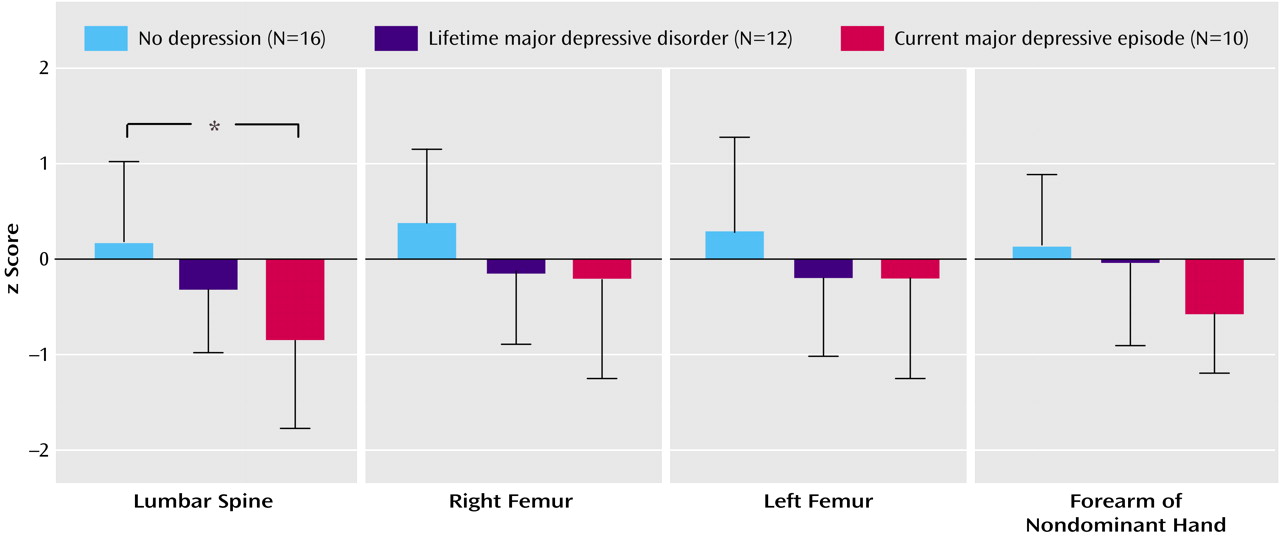
Bone mineral density expressed as a z score was significantly lower in patients with borderline personality disorder plus current major depressive episode at the lumbar spine (t=–2.9, df=28, p<0.02) and at the forearm of the nondominant hand (t=–2.8, df=28, p<0.03) than in the healthy reference group matched for sex, age, and body mass index. Among the three patient groups with borderline personality disorder, z scores differed significantly (F=4.9, df=2, 34, p<0.02). In patients with borderline personality disorder plus current major depressive episode, z scores were significantly lower at the lumbar spine than they were in patients with borderline personality disorder alone (U=30.5, p<0.02) (
Figure 1).
Osteopenia, defined by a T score less than or equal to one standard deviation below that of the reference population at peak bone mass, was observed at the lumbar spine in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode and one (6%) of 16 patients with borderline personality disorder alone (6%). Osteopenia was also observed at the right femur in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode and one (8%) of 12 patients with borderline personality disorder plus lifetime major depressive disorder. Osteopenia was also observed at the left femur in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode, one (8%) of 12 patients with borderline personality disorder plus lifetime major depressive disorder, and two (12%) of 16 patients with borderline personality disorder alone. Osteopenia was also observed at the forearm of the nondominant hand in two (20%) of 10 patients with borderline personality disorder plus current major depressive episode, two (16%) of 12 patients with borderline personality disorder plus lifetime major depressive disorder, and three (19%) of 16 patients with borderline personality disorder alone.
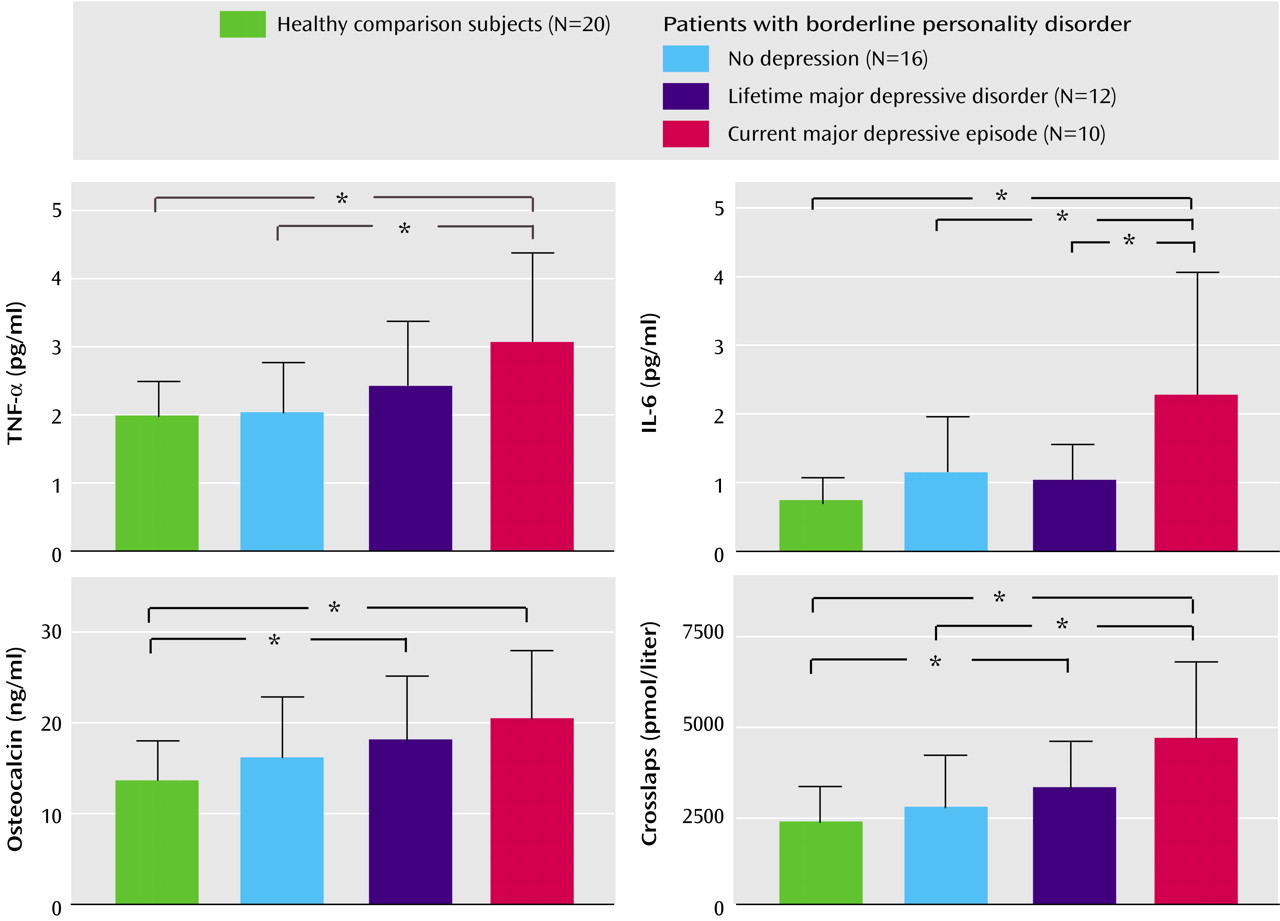
ANOVA revealed significant differences among the three patient groups and the healthy comparison group with regard to age (F=4.2, df=1, 3, p=0.01), fasting cortisol (F=2.8, df=1, 3, p<0.05), osteocalcin (F=3.1, df=1, 3, p<0.04), crosslaps (F=6.4, df=1, 3, p=0.001), TNF-α (F=3.0, df=1, 3, p=0.01), and IL-6 (F=5.3, df=1, 3, p=0.001) (
Figure 2). For further analysis, ANCOVA with the covariate age was performed. The effects of the covariate age were not significant, with p ranging from 0.10 to 0.35. We found a significant group effect for fasting cortisol (F=2.6, df=1, 4, p<0.05), osteocalcin (F=3.0, df=1, 4, p<0.03), crosslaps (F=5.1, df=1, 4, p=0.002), TNF-α (F=3.3, df=1, 4, p<0.01), and IL-6 (F=5.3, df=1, 4, p=0.001).
Post hoc analysis revealed higher concentrations of fasting cortisol in patients with borderline personality disorder plus current major depressive episode than in healthy comparison subjects (p=0.006) and in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder plus lifetime major depressive disorder (p=0.01) (
Table 1). Crosslaps were found to be higher in patients with borderline personality disorder plus current major depressive episode than in healthy comparison subjects (p<0.001), in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder alone (p=0.002), and in patients with borderline personality disorder plus lifetime major depressive disorder than in healthy comparison subjects (p<0.05).
TNF-α was higher in patients with borderline personality disorder plus current major depressive episode than in the comparison subjects (p=0.003) and in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder alone (p=0.006). IL-6 was higher in patients with borderline personality disorder plus current major depressive episode than in healthy comparison subjects (p<0.001), in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder plus lifetime major depressive disorder (p<0.02), and in patients with borderline personality disorder plus current major depressive episode than in those with borderline personality disorder alone (p=0.004).
Regression analysis revealed that TNF-α correlated positively with crosslaps (r=0.322, N=38, p<0.05) but negatively with the z score at the lumbar spine (r=–0.362, N=38, p<0.03). Crosslaps correlated negatively with z scores at the lumbar spine (r=–0.333, N=38, p<0.05). z scores at the right femur correlated negatively with concentrations of serum cortisol (r=–0.33, N=38, p<0.05). Body mass index correlated with z scores at the forearm of the nondominant hand (r=0.416, N=38, p<0.02). No correlations were found between age or body mass index with any of the immunological and hormonal measures examined (data not shown). Addition of medication status to the statistical models had no relevant influence on any of the reported results.
Concentrations of other cytokines and hormones analyzed, including osteoprotegerin, were similar among the four groups (
Table 1). Serum concentrations of IFN-γ and IL-2 were beneath the detection limit in all four groups. White and red blood cell counts, serum electrolytes, and the concentrations of phosphate, magnesium, insulin, thyroid hormones, and prolactin were also similar among the groups (data not shown).
Substance-related disorders were found in eight, posttraumatic stress disorder in 13, and bulimia nervosa in 20 of the 38 patients. ANCOVA revealed no effect of these present or past comorbid disorders on either bone mineral density or the z scores or on the hormonal and immunological data (data not shown).
Discussion
Our data demonstrate low bone mineral density at the lumbar spine in young female patients of normal weight or overweight who had comorbid borderline personality disorder and current major depressive episode. An important aspect of our study is the relative youth of this group of patients (their mean age was 24 years). Earlier studies examining osteoporosis and major depressive disorder were carried out in older groups of patients whose mean ages ranged from 41 to 75 years
(3). Moreover, we found high values for serum crosslaps, which are a marker for osteoclastic activity, and high values for osteocalcin in these patients, indicating high bone turnover.
Patients with borderline personality disorder and a lifetime history of major depressive disorder had nonsignificantly lower bone mineral density than normal, and patients with borderline personality disorder but no history of major depressive disorder had z scores similar to those of the reference group. Our data suggest that the pathophysiological process associated with acute major depressive episode may contribute to the bone loss observed in our patient group. Our data do not support the hypothesis that the pathophysiological process associated with borderline personality disorder per se may lead to a substantial alteration of bone metabolism.
Another finding is that of higher concentrations of TNF-α, IL-6, and fasting cortisol in the patients with borderline personality disorder plus current major depressive episode. Our findings are in accord with those of other researchers who found higher concentrations of proinflammatory cytokines and a dysregulation of the HPA system in patients with current major depressive episode (reviewed in references
7,
10). However, these studies were not stratified for comorbid personality disorders.
The correlation of TNF-α with serum crosslaps and low bone mineral density (expressed as z scores) points to a possible role in the pathophysiological process underlying the bone loss in this group of depressive patients. Bone tissue is continuously rebuilt in a coordinated process of osteoclastic resorption and osteoblastic bone mass increase
(23). Enhanced bone resorption has been associated with an inappropriate osteoclast activation
(24,
25). Several factors, including high concentrations of glucocorticoids and proinflammatory cytokines such as TNF-α and IL-6, have been shown to activate osteoclastic cells in vivo and in vitro. It therefore seems reasonable to hypothesize that the higher levels of serum cortisol and proinflammatory cytokines in patients with borderline personality disorder plus current major depressive episode may play a role in the observed decrease in bone mineral density.
Since the concentrations of osteoprotegerin were similar among the groups and the osteoblastic marker osteocalcin was higher in patients with borderline personality disorder plus current major depressive episode, our findings suggest that the bone loss observed in patients with borderline personality disorder plus current major depressive episode may mainly be attributed to osteoclastic activation rather than to an inhibition of osteoblastic cells. A negative correlation between serum TNF-α and bone mineral density has also been reported in patients suffering from postmenopausal osteoporosis and in healthy adolescent girls
(26,
27).
Other disease- and medication-related processes, such as impaired fluid and electrolyte balance, dietary and vitamin D deficiency, decreased exercise, and decreased exposure to sunshine may play a role in bone loss
(5,
6). Cross-sectional assessment, however, shows that none of the available studies reported them as mediating factors for low bone mineral density in depression. Our data show similar concentrations of parathormone, estradiol, and 1,25-hydroxy-vitamin D in all groups examined. These findings do not support the hypothesis that a deficiency of vitamin D or estradiol or alterations of the parathormone metabolism may be underlying causes of bone loss in our patients.
There are several limitations to our study. First, the small number of patients in each group limits the interpretation of the negative results and increases the risk of spurious results in the comparison of multiple measures. Second, there was no comparison group with major depressive episode and another comorbid personality disorder. This makes it difficult to differentiate whether the reported alteration of bone density is an effect of depression alone or the result of an interaction between depression and borderline personality disorder or its respective risk factors. A third limitation is the restricted ability of the study to control for potential confounding factors like medication or exercise. Studies of alterations in cytokine concentrations in depressed patients during antidepressant treatment
(13,
28) showed a decrease of proinflammatory cytokines (TNF-α and IL-6) after treatment with an SSRI or tricyclic agents. In another cross-sectional study
(29), no influence of previous antidepressant treatment was observed on the concentrations of TNF-α and IL-6 at admission to a hospital. Furthermore, in a longitudinal study
(30), the SSRI paroxetine was shown to leave concentrations of several cytokines (including TNF-α, IL-6) unchanged.
TNF-α and IL-6 have been reported to increase during exercise
(31). Patients with major depressive disorder tend to reduce their physical activity. We are not aware of any study attributing the changes in proinflammatory cytokines in depression to changes in exercise. Taken together, these findings and observations suggest that the concentrations of TNF-α and IL-6 may have been underestimated because of confounding factors in our patient groups. However, we cannot completely exclude that previous medication or exercise may have contributed to the observed findings.
In summary, young women with comorbid borderline personality disorder and current major depressive episode constitute a group at risk of developing osteoporosis. Possible pathophysiological processes include hypercortisolism and an up-regulation of the proinflammatory cytokines IL-6 and TNF-α.