Ephedra is a plant extract also known as
ma huang. During the 1990s, it became popular as a weight loss and exercise enhancement aid. Its active component, ephedrine, is a CNS stimulant
(1,
2); its immediate effects are attributable to stimulation of dopamine release. Ephedrine is defined as a mixed sympathomimetic agent that acts by enhancing the release of norepinephrine from sympathetic neurons and by stimulating alpha and beta adrenergic receptors
(1). This combination of adrenergic and dopaminergic effects leads, in the short term, to improved mood and heightened alertness with decreased fatigue and a lessened desire for sleep. At higher doses, the release of norepinephrine causes anxiety, restlessness, and insomnia. Prolonged use of ephedra may lead to neurotoxicity, resulting in depletion of brain monoamines
(3). Neurotoxicity may result in psychosis, and the structural similarity of ephedrine to amphetamine
(4) raises concern about possible abuse. In addition, ephedra herbal products generally contain caffeine.
Use of ephedra-containing products recently came under scrutiny because of various high-profile adverse events
(5). In 2002, the U.S. Health and Human Services Secretary requested that we synthesize available evidence regarding the efficacy and safety of ephedra
(6). Subsequently, the U.S. Food and Drug Administration (FDA) proposed a ban on the sale of herbal ephedra products in December 2003.
A recent meta-analysis of randomized clinical trials of ephedra and ephedrine for weight loss or improved athletic performance
(7) calculated that ephedra users had 3.64 times the odds of psychiatric symptoms, such as euphoria, neurotic behavior, agitation, depressed mood, giddiness, irritability, and anxiety compared to placebo. However, too few patients have been studied in randomized trials, even taken together, to detect adverse events that occur at a rate of less than 1 per 1,000.
Although there are numerous reports of psychiatric reactions associated with ephedrine-containing bronchial medications
(8), few case reports of psychiatric reactions associated with herbal ephedra can be found in the literature. This article describes data on serious psychiatric adverse events reported to the FDA. Case reports are useful to establish the
potential for a causal relationship; they cannot be considered, except in extraordinary circumstances, to be conclusive evidence of a cause-and-effect relationship.
Method
We reviewed all 1,820 FDA MedWatch reports of adverse events concerning herbal ephedra as of Sept. 30, 2001. Psychosis, mania or severe agitation, severe depression, hallucinations, delusions, suicide attempts, paranoia, and violent behavior were considered serious psychiatric events by our experts. Events such as jitteriness, insomnia, agitation, nervousness, and irritability were reported frequently but were not considered serious enough to fall within the scope of our study. A data abstraction form was developed to collect information on psychiatric, medical, and substance use history, adverse event data, treatment, and various other relevant items. A psychologist and a psychiatrist with expertise in addiction reviewed all reports independently, with differences resolved by consensus. Selected reports of adverse events were reviewed a third time.
All FDA reports contained an official one-page MedWatch form. However, other documents included in the reports, such as medical records, laboratory work, or letters from family members, varied widely. The FDA reports were categorized based on the amount of information supporting a causal relationship. Cases were defined as “sentinel events” if there was no history of psychiatric problems and the patient did not report use of or test positive for other substances known to cause similar symptoms. Evidence of a sentinel event had to include documentation that an adverse event had occurred and that an adequate investigation had excluded other potential causes. Sentinel events did not stipulate that the product was taken within a specific time frame because complications may be caused by cumulative use.
Results
Fifty-seven serious psychiatric events were reported to the FDA. Thirty-two (56.1%) of these cases included reports of psychosis. Other common adverse events (not mutually exclusive) were severe depression (31.6%), mania or severe agitation (26.3%), hallucinations, sleep disturbance, and suicidal ideation (22.8% each). Of the 55 cases for which gender was reported, 60% were women. The patients ranged in age from 13 to 57 (mean=31). Most patients (59.6%) had been using ephedra for more than 2 months. Five patients (8.6%) either self-reported or were diagnosed as being addicted to the products.
In almost all cases, the patients were told to discontinue ephedra use. Ten events involved physical harm to self or others; five events resulted in legal action because of criminal behavior. Twenty-six (45.6%) of the 57 adverse events resulted in hospitalization. At least six hospitalizations were involuntary.
Of importance, two-thirds of the 57 cases involved patients with preexisting psychological/psychiatric conditions and/or use of other mood-altering medications or illicit substances. (Still, only one patient had a history of psychosis.) Thirteen had a documented history of depression; 12 were taking selective serotonin reuptake inhibitors at the time of the event. There were four nonlethal suicide attempts among teenagers; each reported taking the product just one time. In these cases, up to 50 times the recommended dose was ingested. Nine patients had a documented history of substance abuse. Other patient case histories included premorbid eating disorders, anxiety, attention deficit hyperactivity disorder, bipolar disorder, post traumatic stress disorder, and borderline personality disorder.
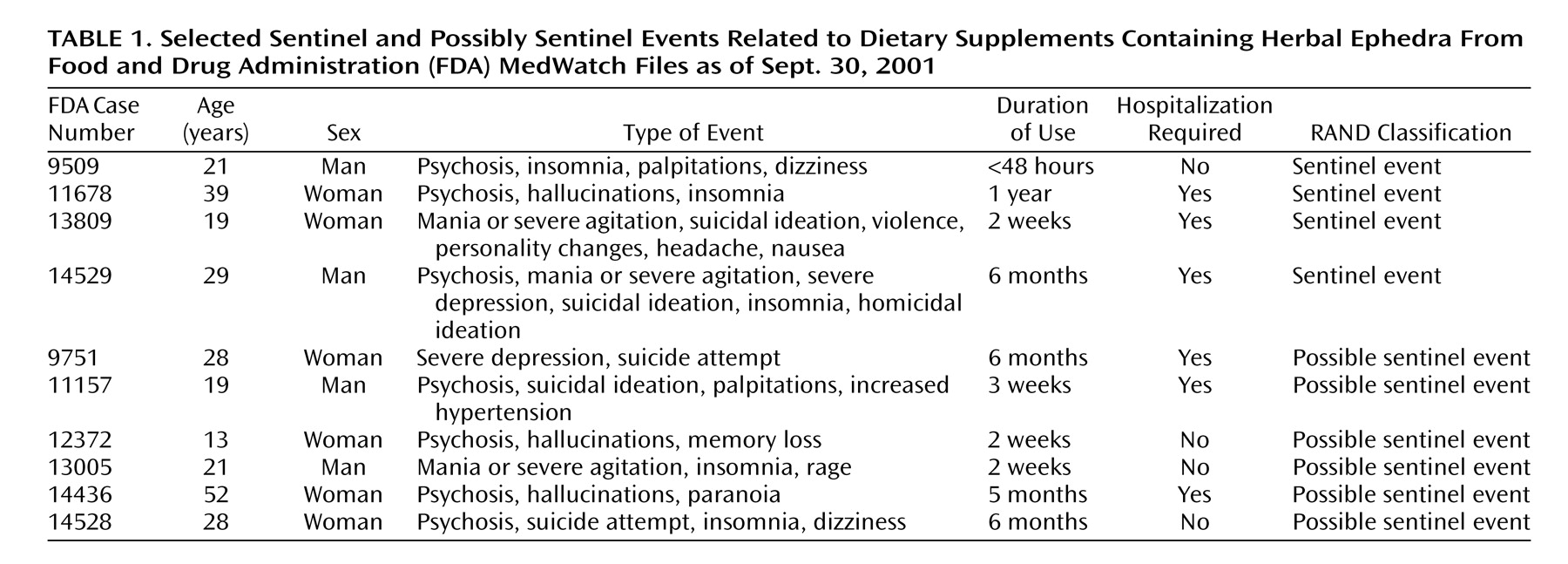
The majority of the reports were insufficiently documented to make an informed judgment about a relationship between ephedra use and the event. Four cases were classified as sentinel events, and an additional six cases were classified as
possible sentinel events because of the limited information in the case report files about psychiatric and medical history (
Table 1).
Three sentinel and three possibly sentinel cases required psychiatric hospitalization. In one case (number 14529), the symptoms recurred twice more after resumed use. Two sentinel and three possibly sentinel cases involved symptom onset within the first 3 weeks of use, while the other cases involved long-term administration.
Discussion
We report on previously unpublished FDA data to alert mental health providers to issues surrounding the use of ephedra dietary supplements. The most frequent psychiatric adverse event reported was psychotic symptoms, often requiring hospitalization. In many cases, there was limited developmental or family history available to assess a biological or genetic predisposition to thought disorders. Most cases described psychotic symptoms in individuals in their teens and 20s, when the onset of psychotic disorders independent of ephedra use is more likely. However, a predisposition does not obviate a role for ephedra in symptom development. The role of stimulants as a cause of psychosis or as a factor increasing susceptibility to the development of chronic or transient psychosis is supported in the literature
(3,
4,
8,
9). Further support for the role of ephedra was the rapid resolution of psychiatric symptoms upon cessation of ephedra use in most sentinel or possibly sentinel cases.
An important limitation of our findings is that most ephedra supplements also include caffeine; therefore, we cannot determine the contributory effect of caffeine. We also cannot estimate the prevalence of psychiatric reactions to ephedra. Mandatory reporting systems do not exist, and a U.S. Inspector General report estimated that surveillance systems probably detect less than 1% of reactions to supplements
(10). In 2003, the FDA proposed placing a telephone number on product labels for reporting adverse events; since then, a ban on ephedra dietary supplements has taken effect.
Our findings are another piece of evidence in addition to evidence of minor psychiatric symptoms found in randomized clinical trials
(7). Although firm conclusions regarding the severe psychiatric effects of ephedra have not been established, taken together, these pieces of evidence raise concern that ephedra may be related to such symptoms. Clinicians should be aware of this.