Several maternal infections have been associated with an elevated risk of schizophrenia in offspring
(1–
3). Evidence suggests that infections known to cause congenital CNS anomalies in humans, including rubella
(4), herpes simplex
(5), polio, and varicella-zoster virus
(1), might be related to the risk of schizophrenia. Toxoplasmosis is also associated with maldevelopment of the CNS, and ecological data have led to the suggestion that this organism might be involved in the etiology of schizophrenia
(6). Therefore, we used serological methods to investigate whether maternal exposure to toxoplasmosis is associated with an increased risk of schizophrenia in adult offspring.
Toxoplasma gondii, the cause of toxoplasmosis, is a ubiquitous intracellular parasite
(7,
8). When primary infection occurs during pregnancy, the offspring have a markedly increased risk of CNS congenital abnormalities, including microcephaly, hydrocephalus, mental retardation, convulsions, cerebral calcifications, and chorioretinitis
(7–
9). Delayed neurologic sequelae, including lower IQ, retarded psychomotor development, and sensorineural deafness, have also been demonstrated in subjects who were exposed in utero, even among those with subclinical infection during the neonatal period
(7,
8).
Method
Description of the Cohort
The Prenatal Determinants of Schizophrenia Study has been described previously
(11) and will be only briefly reviewed here. The cohort members were enrolled in the Child Health and Development Study
(12), which took place from 1959 to 1967. The Child Health and Development Study recruited nearly every pregnant woman under obstetric care from the Kaiser Foundation Health Plan in Alameda County, Calif. The 19,044 live-born offspring of these women were automatically enrolled in the Kaiser Foundation Health Plan. The Child Health and Development Study collected data from maternal medical records, maternal interviews, and other sources.
The Prenatal Determinants of Schizophrenia Study cohort consisted of the 12,094 live births who belonged to the Kaiser Permanente Medical Care Plan between January 1, 1981 (the year in which computerized registries became available) and December 31, 1997. The subjects who remained in the Kaiser Permanente Medical Care Plan and the subjects lost to follow-up were similar to one another on most maternal and paternal characteristics
(11), and the vast majority of individuals who left Kaiser Permanente Medical Care Plan did so before age 10
(11).
Collection of Maternal Sera
Maternal serum samples were obtained during pregnancy for virtually all subjects, were frozen immediately, and were archived at –20°C in a single repository. All specimens were uniformly handled and stored in accordance with a strict protocol.
Diagnosis of Schizophrenia Spectrum Disorders
The outcome was schizophrenia and other schizophrenia spectrum disorders, defined from previous studies
(13) as any of the following: schizophrenia, schizoaffective disorder, delusional disorder, psychotic disorder not otherwise specified, and schizotypal personality disorder. Case ascertainment involved three steps: 1) ascertainment of potential cases from computerized records, 2) chart review of potential cases to confirm eligibility for assessment, and 3) diagnostic interview (or chart review) and consensus diagnosis. Case ascertainment was conducted by a computerized record linkage between the Child Health and Development Study and Kaiser Permanente Medical Care Plan identifiers by using inpatient, outpatient, and pharmacy registries. Subjects from the hospital registry were screened for potential schizophrenia spectrum disorders based on diagnoses of ICD-9 295–299 and psychiatrist review of all psychiatric and medical records. Patients from the outpatient registry screened positive if they were assigned ICD-9 diagnoses of 295, 297, 298, or 299. Subjects from the pharmacy registry screened positive based on a history of antipsychotic treatment.
There were 13 deceased subjects among those who screened positive for potential schizophrenia spectrum disorders (N=183). Among the 170 remaining potential subjects with schizophrenia spectrum disorders, 146 (86%) were contacted to schedule a diagnostic interview.
Clinicians with at least a master’s degree in a mental health field who were trained to reliability administered the Diagnostic Interview for Genetic Studies to potential subjects with schizophrenia spectrum disorders
(14). Consensus of three experienced research psychiatrists was used to obtain DSM-IV diagnoses based on a review of the Diagnostic Interview for Genetic Studies narrative and medical records and discussions with the interviewer. The Diagnostic Interview for Genetic Studies was completed by 107 (73%) of the 146 contacted potential subjects with schizophrenia or schizophrenia spectrum disorders. For the 76 potential subjects who were not interviewed, chart reviews by experienced clinicians were conducted; all diagnoses were confirmed by a research psychiatrist. These procedures yielded a total of 71 subjects with schizophrenia spectrum disorders, 44 of whom received the Diagnostic Interview for Genetic Studies and 27 of whom were diagnosed by chart review. Among these 71 subjects with schizophrenia spectrum disorders, 64 had available prenatal sera. The diagnoses of these subjects were schizophrenia (N=38), schizoaffective disorder (N=15), delusional disorder (N=1), schizotypal personality disorder (N=5), and other schizophrenia spectrum psychosis (N=5).
All subjects in the Prenatal Determinants of Schizophrenia Study provided written informed consent for human investigation. The study protocol was approved by the institutional review boards of the New York State Psychiatric Institute and the Kaiser Foundation Research Institute.
Laboratory Assay
All of the assays were performed with researchers blind to case/comparison status in the Toxoplasma Serology Laboratory at the Palo Alto Medical Foundation Research Institute, the
Toxoplasma reference laboratory for the United States
(15), under the direction of Dr. Jack Remington.
Three assays were used in the present study
(15). The first two concern the assessment of
Toxoplasma IgG antibody titer. In accordance with established practice, samples were first screened for the IgG antibody titer by using the screen agglutination test. Then, to definitively establish the presence of
Toxoplasma IgG antibody, the Sabin-Feldman dye test
(16), the reference standard for the serological detection of
Toxoplasma antibody
(7), was performed in the samples that screened positive on the agglutination test.
To examine whether recent infection with toxoplasmosis occurred in our samples, we assayed for
Toxoplasma IgM antibody using the double-sandwich enzyme-linked immunosorbent assay (IgM ELISA)
(17). Given the extremely low likelihood that positive IgM antibody would be found in a sample with a negative antibody result on the IgG screening agglutination test, the IgM ELISA was performed only on subjects who screened positive in the agglutination test.
Categorization of Exposures
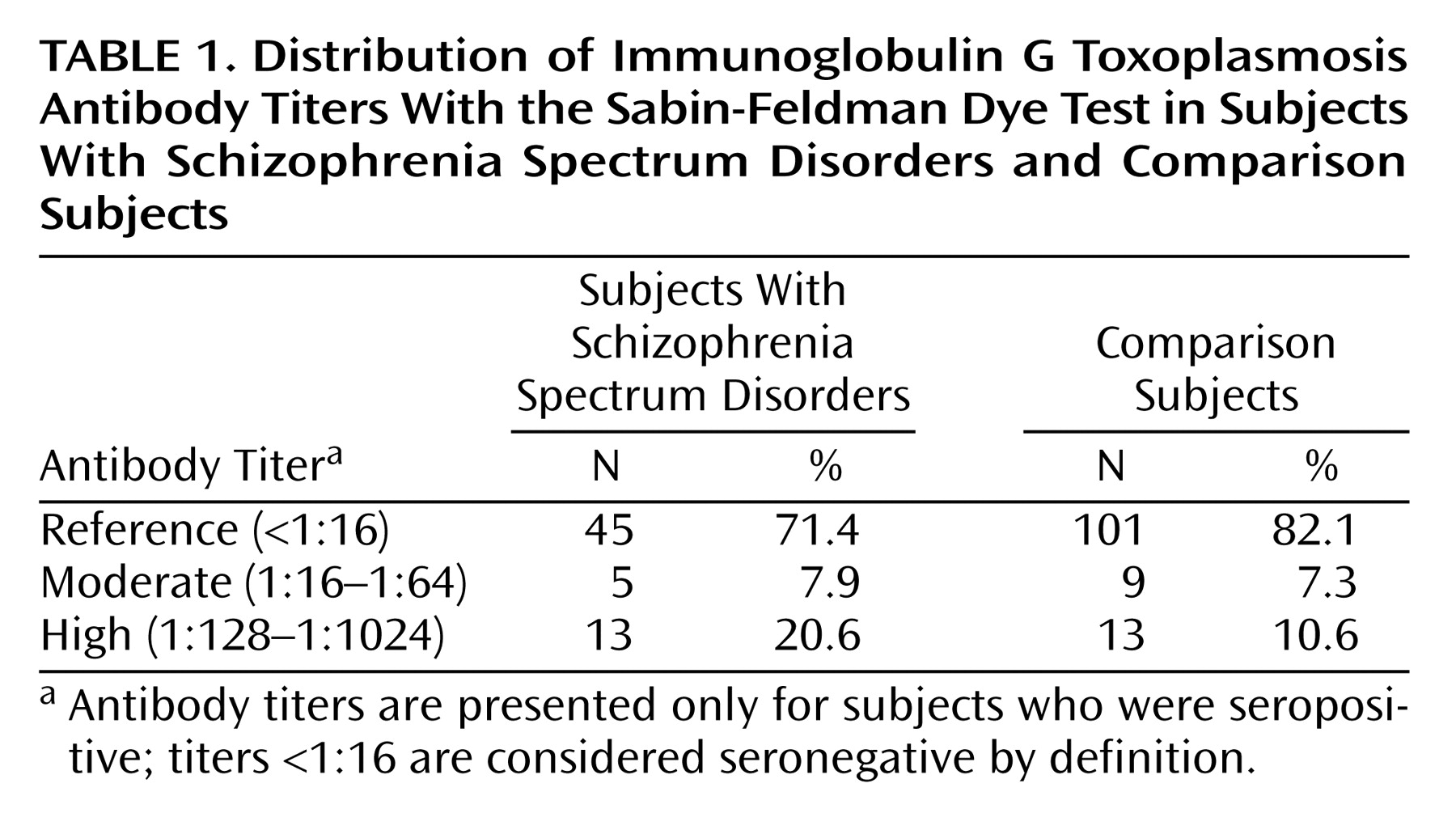
Following established practice, the screen agglutination test results for Toxoplasma IgG were categorized as a dichotomy (positive/negative).
The Sabin-Feldman dye test
(16) yields IgG antibody titer results in serial twofold dilutions. All dye test IgG titers <1:16 are considered negative. Given the lack of clear precedents in the literature for the classification of
Toxoplasma IgG antibody titers, we categorized the subjects with positive IgG titers based on the distribution of the titers in our sample. “High” titer was defined as a
Toxoplasma IgG antibody titer of ≥1:128; this category represented approximately the highest 10th percentile (10.5%) of IgG titers for comparison subjects in our study group. The moderate titer group was defined as an IgG antibody titer of 1:16–1:64 and consisted of the remaining subjects with positive IgG antibody titers. This classification strategy provided sufficient subject numbers in each of the exposure groups to permit a meaningful analysis of the data, while also allowing us to examine the effect of different magnitudes of antibody titer on the risk of schizophrenia spectrum disorders. The reference category consisted of subjects with negative IgG antibody titers.
In accordance with the methods used by the Toxoplasma Serology Laboratory at the Palo Alto Medical Foundation Research Institute
(17), IgM ELISA antibody titer values >1.9 were considered positive, values from 1.7 to 1.9 were equivocal, and values ≤1.6 were negative.
Analytic Strategy
The analysis was based on a nested case-control design
(18) in which the comparison subjects for each case are selected to represent the population at risk when the case was ascertained. In the Prenatal Determinants of Schizophrenia Study, cases were ascertained on the first date of medical attention for schizophrenia spectrum disorders.
Eligible Subjects With Schizophrenia Spectrum Disorders
Among the 71 subjects with schizophrenia spectrum disorders, 64 had at least one available prenatal serum sample; 58 (90.6%) of these 64 subjects had either schizophrenia or schizoaffective disorder. The last serum sample available for each pregnancy (late third trimester or perinatal) was used. This provided the greatest opportunity to detect
Toxoplasma infection if it occurred at all during pregnancy. Even if exposure occurred in early pregnancy, it was unlikely to have been missed by our assay method because
Toxoplasma IgG antibodies generally remain elevated for many months or years after infection
(7).
Eligible Comparison Subjects
Eligible comparison subjects (N=10,768) were selected from offspring without schizophrenia spectrum disorders in the Prenatal Determinants of Schizophrenia Study cohort after excluding siblings of subjects with schizophrenia spectrum disorders, subjects with major affective disorders, and subjects without prenatal sera.
Matching Procedure
Matching by a nested case-control design ensured that each case and its corresponding comparison subject were followed for equal lengths of time from birth until first treatment of the case. Comparison subjects were matched to subjects with schizophrenia spectrum disorders on membership in the Kaiser Permanente Medical Care Plan at the time the case was ascertained, date of birth (±28 days), gender, and number and timing (±28 days) of the first maternal blood sample taken during the index pregnancy
(2,
11).
To conserve the sera, two comparison subjects were selected at random from the pool of potential matched comparison subjects for each case and were further matched on gestational age by requiring that they were drawn within 42 days of the serum sample of the case. This selection process resulted in 124 matched comparison samples (60 sets with 1:2 matching and four sets with 1:1 matching). A comparison in a 1:1 matched set was eliminated from the analysis because of an insufficient quantity of serum, resulting in 63 subjects with schizophrenia spectrum disorders and 123 comparison subjects (60 sets matched at 1:2 and three sets matched at 1:1). Gestational ages (in days) of sera from subjects with schizophrenia spectrum disorders (mean=271.7, SD=24.8) and comparison subjects (mean=275.3, SD=26.3) did not differ significantly from one another (t=–0.67, df=184, p=0.51).
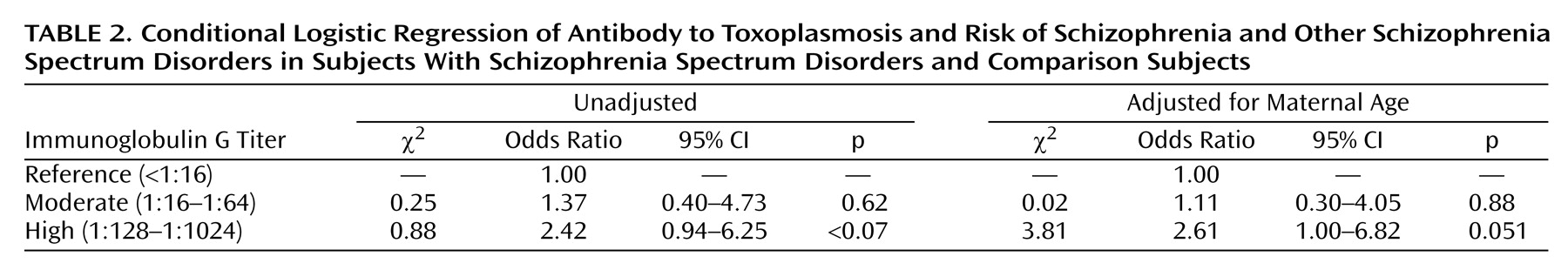
Appropriate to the nested case-control study design, point and interval estimates of odds ratios were obtained by fitting conditional logistic regression models for matched sets
(19). We first tested the relationship between high maternal
Toxoplasma IgG and risk of schizophrenia spectrum disorders. We then examined whether moderate
Toxoplasma IgG was associated with schizophrenia spectrum disorders. Statistical significance was judged at α=0.05.
After first obtaining unadjusted estimates of the association between Toxoplasma IgG and schizophrenia spectrum disorders, we then assessed the following covariates as potential confounders: maternal age (<35 [reference], ≥35), maternal ethnicity (Caucasian [reference], African American, other), maternal socioeconomic status, defined as maternal education (<high school, high school only [reference], some college/college graduate), and gestational age of the serum sample (in days after last menstrual period).
Discussion
To our knowledge, this is the first report of an association between elevated maternal IgG antibody to Toxoplasma and the risk of schizophrenia. This finding was revealed by using prospectively collected and archived prenatal serum specimens in a well-characterized birth cohort. Although the finding fell slightly short of statistical significance, these facts lend credence to the result.
The relationship was observed only for the high category of IgG antibody titer. It should be noted, however, that our definition of “high” titer was based on the maternal titers relative to our sample, rather than those found in the mothers of infants with congenital toxoplasmosis, in which elevated maternal IgM titers and/or very high IgG titers are typically observed. In the present study, no subjects had elevated maternal IgM titers, and very high IgG titers were rare. Thus, antibody titers in the “high” exposure group in our cohort may be consistent with subtle adverse developmental effects on the brain, such as those that could influence the pathogenesis of schizophrenia.
As reviewed in our introduction, toxoplasmosis during pregnancy is a known cause of congenital abnormalities in offspring
(10,
11,
20,
21). Although most of these studies are based on direct observation of the parasite, elevated maternal IgM, or persistently elevated IgG in infant serum along with elevated IgG in maternal serum
(9), some studies have examined whether a single elevated maternal
Toxoplasma IgG antibody titer is associated with harm to the offspring. Sever et al.
(10) showed that the 7-year-old offspring of mothers with elevated IgG
Toxoplasma antibody titers had substantial increases in microcephaly, low IQ, and bilateral deafness. Effects were also observed, although at smaller magnitudes, for coordination problems, visual impairment, and extraocular movement abnormalities.
Prenatal exposure to toxoplasmosis is a plausible risk factor for schizophrenia. The parasite has a predilection for the developing fetal brain
(7,
8) and results in a similar array of congenital abnormalities as rubella and other pathogens that have been implicated in schizophrenia
(1,
4,
5). Postmortem and neuroimaging studies in newborns with congenital toxoplasmosis have revealed several CNS abnormalities, including enlargement of the third and lateral ventricles secondary to hydrocephalus, and intracranial calcifications, frequently of the basal ganglia, choroid plexus, and meninges. Although these findings differ in severity, and to some degree in type, from those in schizophrenia, radiological studies have not been conducted on children who were exposed in utero to toxoplasmosis but who did not have the stigmata of congenital toxoplasmosis at the time of birth. It has been shown, however, that 40%–70% of these asymptomatic newborns later developed neurocognitive and neuromotor abnormalities that resemble those found in children who were later diagnosed with schizophrenia
(8). These sequelae include retarded mental and psychomotor development, mildly decreased IQ, coordination problems, neurologic soft signs, and stereotypies
(7,
10).
Because antibody titers to
Toxoplasma IgG may remain elevated for significant periods of time, an increase in IgG antibody may reflect an active primary infection, reactivation of infection, or a persistent immune response to a dormant infection. First, we discuss active primary infection. In the present study, none of the serum samples in our cohort tested positive for IgM-specific
Toxoplasma antibody, the most robust indicator of recently acquired infection
(7). In a previous birth cohort study conducted during the same years as the Child Health and Development Study, the prevalence of primary
Toxoplasma infection during pregnancy was low
(10), consistent with subsequent studies
(7). These results indicate that primary infection is unlikely to account for the observed finding.
Second, we consider reactivation of a previous infection. Following acute
Toxoplasma infection, the parasites are not completely eliminated by the immune system; rather, they become sequestered as bradyzoites in dormant cysts in affected organs, most commonly the brain, eye, heart, and skeletal muscle
(7,
8). However, the cysts have been known to spontaneously rupture as a result of host immunosuppression or other factors. When this occurs, reactivation of infection is produced by conversion of bradyzoites into tachyzoites, which are released from the cyst, proliferate, and invade cells
(7,
22). The resulting anamnestic response produces elevations of IgG antibody titers. An effect of activated dormant
Toxoplasma microcysts on brain function is suggested by studies demonstrating associations between increased
Toxoplasma IgG antibody and first-episode schizophrenia
(23), personality changes
(24), and cryptogenic epilepsy
(25) in adult patients. The prevalence of reactivated infection is not known.
Toxoplasma is transmitted to the fetus through the placenta
(26). Infection is most often transmitted to the offspring when
Toxoplasma is acquired later in pregnancy, with the highest risk in the third trimester
(26). The proliferating tachyzoites in the fetal CNS and other organs destroy parasitized cells and result in an inflammatory response, leading to anoxia, cell death, and tissue necrosis
(8).
Toxoplasma also increases levels of homovanillic acid and dopamine, which are implicated in the pathogenesis of schizophrenia
(27).
It has also been suggested that
Toxoplasma gondii may result in congenital CNS abnormalities without direct transmission of the parasite to the fetus. This mechanism is proposed to involve toxofactor, a toxin released by
Toxoplasma (28). When administered during pregnancy, toxofactor causes congenital abnormalities, particularly CNS defects, in exposed animals.
It is also possible that the risk of schizophrenia may be increased in the offspring of mothers with dormant
Toxoplasma infection and elevated IgG antibody. Individuals with dormant
Toxoplasma may have elevations of IgG antibody for months or years following the infection
(7). Under this scenario,
Toxoplasma IgG antibody, rather than the organism or a toxic product, may cross the placenta and cause damage to the developing fetal brain. IgG antibodies from women with spontaneous abortion
(29) and from subjects with systematic lupus erythematosus
(30) are known to cause teratogenic effects.
Our findings may help to distinguish between these possible explanations. The level of IgG antibody titer is generally correlated with both the severity and recency of infection. Thus, antibody titers in the “high” category are more likely to be associated with a current or recent reactivated infection than titers in the “moderate” category, which have a greater probability of reflecting dormant infection.
Given associations between prenatal exposure to other infectious agents and the risk of schizophrenia
(1,
2), it also possible that an alteration of maternal immune status may have accounted for the findings. We aim to test this hypothesis in future work.
In a previous study from the Collaborative Perinatal Project, no association was found between antibody to
Toxoplasma and the risk of adult psychosis
(5). The group size of this study was small, however, and there was greater heterogeneity of psychotic disorders than the present study. Furthermore, the previous study quantified IgG antibody by solid-phase enzyme immunoassay rather than by the Sabin-Feldman dye test, the reference standard because of its high sensitivity and specificity
(7).
Limitations
The sera in the present study had been frozen for over 30 years, raising the possibility that storage for this period of time may have altered the
Toxoplasma antibody levels. However, this factor appears unlikely to have had an appreciable impact on our results for several reasons. First,
Toxoplasma antibody levels are generally stable in frozen stored sera. Second, the seroprevalence of toxoplasmosis in comparison subjects was 17.9%, similar to the 17.5% seroprevalence found in a large previous study of toxoplasmosis in reproductive-age women in the United States
(31). Third, we matched the comparison subjects to subjects with schizophrenia spectrum disorders on the date of birth and gestational timing, and the samples were uniformly handled and stored, indicating that storage time should not have biased the associations.
Second, we should consider the potential impact on our findings of associations between determinants of toxoplasmosis and factors related to maternal lifestyle or health. Toxoplasmosis is generally acquired by eating raw or undercooked meat containing
Toxoplasma gondii tissue cysts, by ingesting oocysts from soil through activities such as gardening or eating unwashed vegetables or fruits, or possibly by exposure secondary to changing cat litter boxes
(31). It is conceivable that mothers of future patients with schizophrenia, in relation to mothers of comparison subjects, were more likely to engage in these activities. It is also possible that factors related to determinants of toxoplasmosis and to maternal lifestyle and health in mothers of schizophrenia patients may have confounded the observed association. Maternal or family history of schizophrenia might be considered such a factor. We have not yet acquired sufficient data on family history of schizophrenia spectrum disorders to examine potential confounding by family history. There is no clear reason, however, to postulate that maternal toxoplasmosis should be related to maternal or other family history of schizophrenia after adjustment for potential demographic risk factors. Even if such a relation exists, the effect of these factors would need to be very substantial to account for the observed associations, and we did adjust for the potential confounders available in our data set (age, social class, or ethnicity); nonetheless, we cannot entirely rule out this possibility.
Third, the finding was marginally significant, and the group size was modest. Thus, independent replication of this result is essential.