The use of control subjects given placebo in clinical trials is associated with ethical problems, especially in cases where effective treatment is available
(1,
2) and where progressive diseases involve potential deterioration that is likely to be irreversible. In Europe, however, granting a license to study treatment of bipolar disorder, manic episode, and bipolar depression and the prevention of manic/depressive episode is conditioned on demonstration of efficacy in comparison with placebo
(3).
Patients with bipolar disorder are at high risk for committing suicide; the estimated rate is 400/100,000 person-years of exposure, compared with the international general population average of 17/100,000 person-years of exposure
(4,
5). The rate of suicide attempts is approximately 2,000/100,000 person-years of exposure in patients with bipolar disorder
(6,
7).
Recently, Goodwin et al.
(8) reported that anticonvulsant treatment was associated with higher rates of suicide and suicide attempts than was lithium among patients treated for bipolar disorder for varying amounts of time. This finding is consistent with others noting that lithium, the gold standard therapy in bipolar disorder, may lower suicide risk
(5,
6,
9,
10).
In addition to all other controversial issues concerning the use of placebo, the high risk of suicide in bipolar disorder and the possible suicide protection of lithium are arguments against the conduct of studies with a placebo group in this disorder
(11).
The aim of this study is to investigate whether the risk of suicide and attempted suicide is indeed greater in placebo groups in studies of the treatment of acute manic episode and the prevention of manic/depressive episode in order to determine if this would be an argument against the conduct of such studies.
Method
All double-blind, placebo-controlled, randomized trials conducted for the treatment of acute manic episode and for the prevention of manic/depressive episode
(3) that were part of a registration dossier submitted to the Medicines Evaluation Board of the Netherlands between the years 1997 and 2003 were reviewed for committed and attempted suicides. (In 1997, after a long period of time, the first compound for the treatment of bipolar disorder was submitted to the Medicines Evaluation Board.) The duration of the acute manic episode studies varied between 3 and 12 weeks. Prevention studies were included only if they had a minimum duration of 52 weeks.
The Medicines Evaluation Board is the regulatory authority of the Netherlands. To obtain a marketing authorization, pharmaceutical companies are required to submit a dossier to the Medicines Evaluation Board that includes all clinical trials conducted for a drug under development.
Pharmaceutical companies are required to report in their registration dossiers all efficacy and safety results, including suicides and attempted suicides
(12). These dossiers contain studies that may have been published as well as studies that will never be published. The decision of whether to grant a market authorization to a given product is based on the assessment of the complete dossier.
The original studies submitted by the companies to the Medicines Evaluation Board dossiers were selected. All suicides and suicide attempts that occurred during the placebo-controlled phase (as defined in the individual study protocols) of these studies were considered cases. The a priori definition of suicide and suicide attempt in this investigation was the definition that was used in the studies submitted to the Medicines Evaluation Board. Because the dossiers submitted are confidential and are the property of the pharmaceutical companies, the dossiers were made anonymous.
Analyses were based on the intent-to-treat population, including all patients who were randomly assigned to active medication or placebo. Suicides and attempted suicides that occurred after the placebo-controlled phase were not included in the analyses. The incidence of suicide and suicide attempts was estimated for person-years at risk, and the statistical significance of differences between groups was assessed on the basis of a Poisson model.
Results
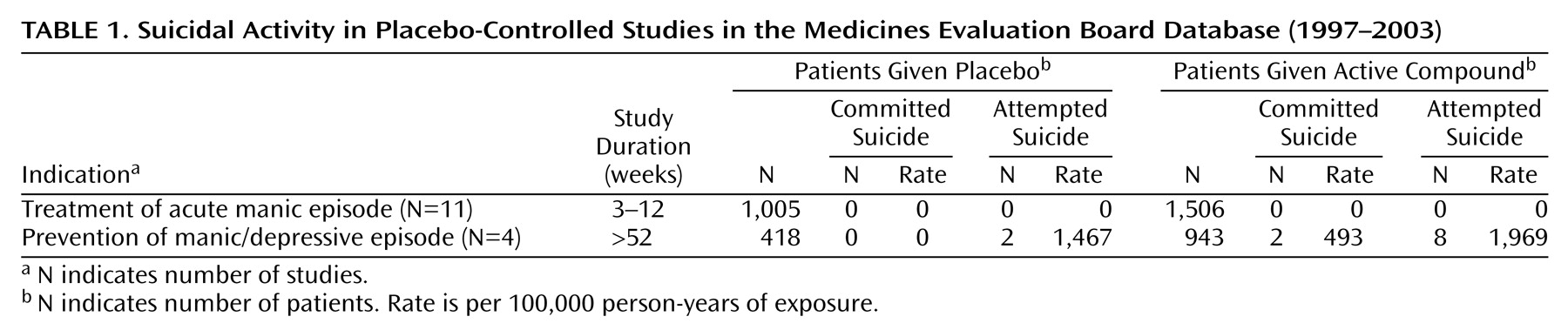
During the period under investigation, 11 placebo-controlled studies for treatment of acute manic episode and four studies for the prevention of manic/depressive episode were submitted to the Medicines Evaluation Board (
Table 1).
A total of 2,511 patients were included in the 11 intervention studies for acute manic episode: 1,506 in the active compound groups and 1,005 patients in the placebo groups. These patients contributed in total 188 person-years of exposure (117 person-years of exposure in the active compound groups and 71 person-years of exposure in the placebo group). The duration of the studies varied from 21 days to 84 days. Six studies had a three-arm design. Lithium was the active comparator in four studies and haloperidol in two. All studies included patients who were hospitalized at the beginning of the study. DSM criteria were used for the diagnosis in 10 studies: DSM-III-R criteria in one and DSM-IV criteria in nine. Suicidal patients were excluded at baseline in seven studies (N=1,685); suicidal patients were not explicitly excluded in the other four (N=826). Exclusion of suicidal patients was done on the basis of the clinician’s evaluation at entry to the study in five studies and based on a rating of 3 or more on item 3 of the Hamilton Depression Rating Scale in the other two.
No suicide occurred while patients were receiving the active medication, the active comparator, or the placebo during the study period.
One suicide occurred 21 days after the completion of the study in a patient from an active treatment group who was using medication at the time of suicide. One fatality occurred in the placebo group: a patient discontinued the placebo treatment 6 days after the start of the study because of insufficient response and died 20 days later as a result of a motor vehicle accident. This accident was coded as injury and not suicide.
One attempted suicide occurred in the placebo group in a patient who decided to withdraw from the study after 2 days of treatment. This patient was treated for 3 weeks with active medication and made a suicide attempt 3 days later. These cases of suicide and attempted suicide that occurred after the placebo-controlled phase of the studies were not included in the analysis.
A total of 1,361 patients were included in the four studies of the prevention of manic/depressive episode: 943 in the active compound groups, contributing 406 person-years, and 418 patients in the placebo groups, contributing 136 person-years. The duration of the studies varied from 52 to 76 weeks. Three studies had a three-arm design with lithium as active comparator; in these studies 258 patients were treated with lithium, contributing 80 person-years. Two studies included inpatients and outpatients, and the other two studies included only outpatients. DSM-III-R criteria were used to establish the diagnosis in one study, and DSM-IV criteria were used in the other three studies. Suicidal patients were excluded at baseline in all studies. In two studies the clinician determined at entry of the study whether the patient was at serious suicide risk. In the other two studies suicide risk was based on a rating of 3 or more on item 3 of the Hamilton depression scale.
Two patients committed suicide in the long-term studies of prevention of manic/depressive episode. Both suicides occurred in active compound groups. The suicide rate in the combined active compound group was 493/100,000 person-years of exposure. Another suicide occurred in the active compound group 3 weeks after the patient dropped out of the study. This patient was not included in the analysis. No suicide occurred in the placebo groups; therefore, no statistical test was possible.
There was a total of 10 attempted suicides. Two occurred in the combined placebo group and eight in the combined active compound group. The incidence rate of suicide attempts per 100,000 person-years of exposure was 1,467 in the placebo group and 1,969 in the active compound group. The differences between the two incidence rates were not statistically significant (likelihood ratio=0.15, df=1, p<0.71, likelihood ratio test).
Two patients who were in the active compound group made a suicide attempt during follow-up, i.e., after discontinuation from the study. These patients were not included in the analysis.
No suicides occurred in the lithium treatment groups. Two suicide attempts were reported in the lithium treatment groups, resulting in a suicide attempt rate of 1,801/100,000 person-years of exposure in the lithium groups.
Discussion
The results presented here indicate no greater risk of suicide among patients with acute manic episode or stabilized bipolar disorder who were treated with placebo compared with the risk of patients who were treated with an active compound under the conditions of the trials. Moreover, the inclusion in the analysis of the suicides and attempted suicides that occurred after the completion of the studies does not change this result. These findings are compatible with findings from depression
(13,
14) and schizophrenia
(15,
16) trials.
Several limitations of this study should be considered when interpreting its results. The first is its limited power, due to the relatively low incidence of suicide and attempted suicide even in this high-risk population (1,467 suicide attempts per 100,000 person-years in the combined placebo groups). Yet it should be kept in mind that the evidence presented here is likely to be the best available because of the extraordinary large number of patients included in the pooled analysis (N=3,872).
A bias that may limit the generalizability of the results is caused by the exclusion in these studies of patients who were at risk for committing suicide
(7,
17–19). However, in the recently reported study of Goodwin et al.
(8) the incidence of suicide was 66/100,000 person-years of exposure in the lithium treated patients and 155/100,000 person-years of exposure in patients given anticonvulsants (mainly divalproex), whereas the suicide rate in the active treatment arms of the placebo-controlled prevention studies of our investigation was 493/100,000 person-years of exposure. This figure indicates that patients with bipolar disorder are at high risk for suicide, even if they are considered nonsuicidal and treated with an active compound.
The studies included in our investigation are part of registration files. Because some studies are not submitted to the Medicines Evaluation Board (e.g., because a company decided to stop further development of the drug) our investigation did not cover all studies conducted in the period 1997 to 2003. However, “negative” studies and new studies that have not (yet) been published were available in the database. Therefore, this information is not likely to be affected by publication bias and is adequate for addressing the current research question.
We believe that the evidence presented gives some indication that placebo treatment does not raise the risk of suicide among patients who are eligible to participate in these kinds of trials.