Medication-free schizophrenia research is an important tool in the scientific community’s quest to understand this devastating illness because this methodology enables researchers to examine the illness through the clearest possible lens. One way to accomplish such research is to recruit medicated participants who go through a medication-free interval before the relevant study procedures and resume treatment after completing the study. Despite obvious advantages, such studies carry with them a number of potentially thorny ethical issues. As one example, it is conceivable that a participant who provides informed consent at the beginning of a study may lose this capacity because of symptom exacerbation when medications are discontinued. If this were to occur, the individual’s ability to assess his or her situation and make an informed decision as to whether to continue in the study or withdraw may become impaired.
Studies have shown that a high percentage of individuals with schizophrenia have adequate decisional capacity to consent to research participation
(1) and that those with reduced capacity can benefit significantly from remedial intervention
(2). However, to our knowledge, there are no published data to indicate whether capacity for informed consent changes during the course of medication-free schizophrenia research. This study was designed to answer this important question and to examine changes in cognitive function and psychiatric symptoms that occur following medication discontinuation in the research setting.
Method
This study was approved by the University of Iowa Institutional Review Board. Written informed consent was obtained from all participants following a thorough discussion of the study. Participants were also required to provide acceptable responses to all questions on the Evaluation to Sign Consent
(3).
Participants included 10 individuals with a DSM-IV diagnosis of schizophrenia (eight men and two women). The patients’ mean age was 32.90 years (SD=11.64), and their mean level of education was 13.80 years (SD=2.69). At enrollment, all participants had already consented to participate in a separate neuroimaging protocol involving an inpatient medication-free period of approximately 2 weeks.
The assessments described here were conducted immediately before the discontinuation of antipsychotic medication and again at the end of the medication-free interval, before reinstatement of medication. The mean medication-free interval was 13.30 days (SD=2.95, range=7–18). Participants were taking the following medications at enrollment: risperidone (N=2), quetiapine (N=2), ziprasidone (N=2), clozapine (N=1), haloperidol (N=1), olanzapine (N=1), and haloperidol plus risperidone (N=1).
Three participants, not included in the group of 10 described here, were enrolled in the study but were subsequently excluded when their medication-free period was terminated in less than 7 days because we had set a minimum 1-week threshold for inclusion. All three of these participants experienced an exacerbation of symptoms shortly following medication discontinuation and, following discussion with their physician, made an informed decision to withdraw from research.
After obtaining consent for this study, the examiner asked each participant to pretend that he or she was a potential candidate for another research study and, together with the participant, read through a detailed informed consent document from a
hypothetical double-blind, placebo-controlled trial of a cognitive-enhancing agent called Synaptoclear. The examiner then administered the MacArthur Competence Assessment Tool for Clinical Research
(4), a structured interview that was customized for the hypothetical study, allowing the examiner to quantify the participant’s decisional capacity on four dimensions: understanding, appreciation, reasoning, and ability to express a choice. Participants who earned fewer than 20 of the 26 possible points on the understanding scale (N=2 at baseline and N=1 at follow-up) were administered a brief educational intervention and then reassessed with the MacArthur Competence Assessment Tool for Clinical Research. Finally, the examiner administered the Evaluation to Sign Consent, a brief structured interview designed to assess the adequacy of participants’ understanding of the hypothetical study.
The Repeatable Battery for the Assessment of Neuropsychological Status
(5) was administered to assess overall level of neuropsychological functioning based on performance in several domains, including immediate memory, visuospatial/constructional, language, attention, and delayed memory. Both Form A and Form B of the Repeatable Battery for the Assessment of Neuropsychological Status were used, in a counterbalanced manner, to minimize practice effects during the follow-up assessment. Negative, psychotic, and disorganized symptoms of schizophrenia were assessed with the Scale for the Assessment of Negative Symptoms
(6) and the Scale for the Assessment of Positive Symptoms
(7).
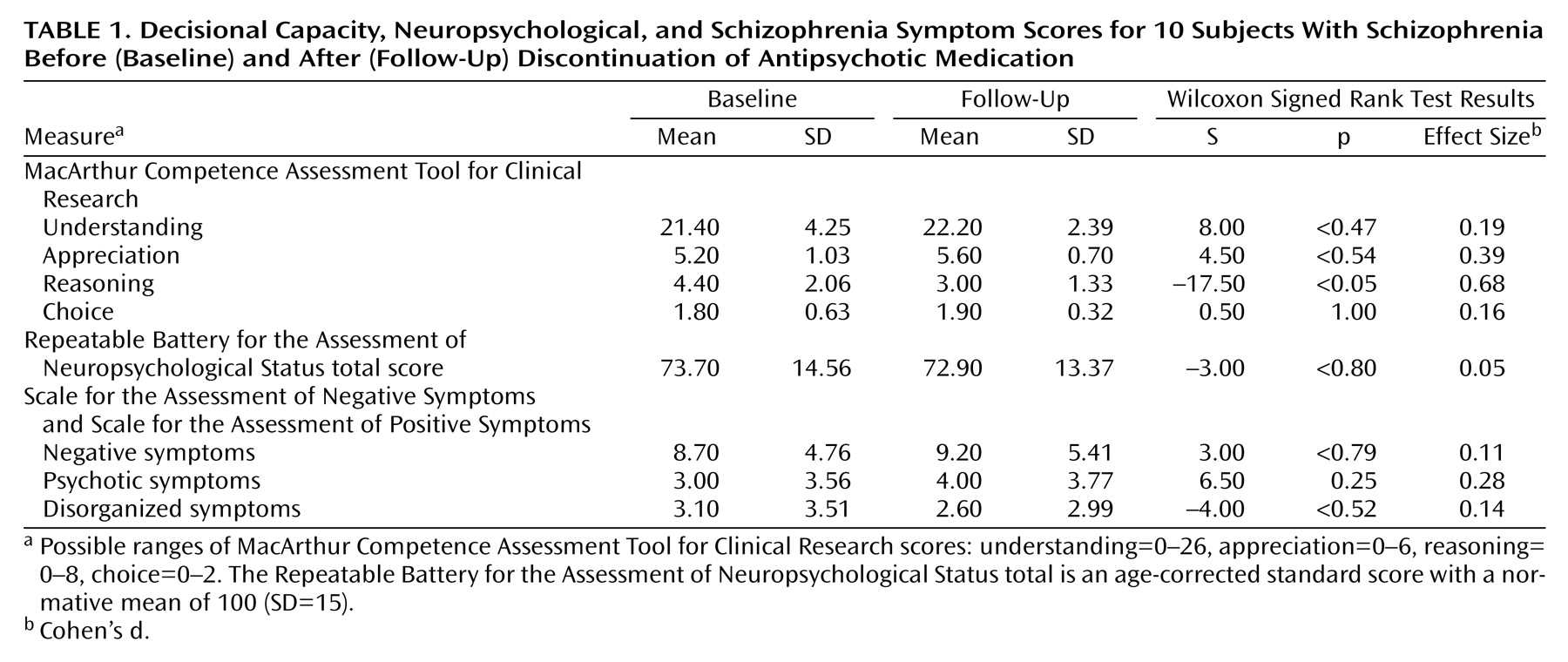
Wilcoxon signed rank tests (with exact p values) were conducted to compare baseline and follow-up scores on the MacArthur Competence Assessment Tool for Clinical Research understanding, appreciation, reasoning, and choice scales. Additional Wilcoxon tests were conducted on an exploratory basis to determine whether there were significant changes in cognitive scores or psychiatric symptoms across the medication-free interval. Because there were only 10 subjects and given the preliminary nature of the study, an alpha level of 0.05 was retained for all tests.
Discussion
We consider these results to be encouraging and highly consistent with less formal observations we have made during the course of our medication-free research. As a group, schizophrenia research participants undergoing a medication-free interval experienced no decline in most aspects of decisional capacity, and in no case did a participant who had adequate understanding of study procedures at baseline subsequently lose this capacity. Additionally, psychiatric symptoms and neuropsychological test scores changed very little for participants completing the medication-free interval.
It is important to reiterate that three additional participants withdrew from the study and resumed medication before day 7 of the medication-free interval due to an exacerbation of symptoms. In all three cases the participant consulted with his or her physician and made what was judged to be an informed decision to withdraw from the study.
The pattern and magnitude of the baseline MacArthur Competence Assessment Tool for Clinical Research scores presented here are consistent (within 0.18 standard deviations of the mean) with the performance of 25 individuals with schizophrenia who participated in our previous study
(1). The issue of concern in the present study, however, is that the participants’ relatively poor reasoning ability declined significantly following medication discontinuation. The reason for this remains unclear. It is possible that this was associated with a cognitive or psychiatric change that was not grossly evident on the instruments used to assess these factors.
Although limited by a small number of subjects and a medication-free interval that could be considered brief by some standards, the current study suggests that individuals with schizophrenia do not show a major decline in decisional capacity during the course of medication-free research. However, additional investigation is needed to shed light on the magnitude and etiology of changes in reasoning ability that may occur following medication discontinuation. A challenging and important aspect of future studies will be the development of remediational interventions aimed at enhancing reasoning ability; at present, most interventions focus on improving basic understanding of a given study’s procedures, risks, and benefits.