Serotonin is thought to play a major role in a number of psychiatric diseases and various symptoms of other psychopathologies
(1). To the extent that serotonin-mediated psychopathology is a life-long pattern of reoccurring episodes of disease with impaired function and distress
(1), it is reasonable to postulate that stability of interindividual differences in central functioning would underlie the long-term risk for serotonin-mediated psychopathological disorders. CSF concentrations of the major serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) are thought to reflect CNS serotonin turnover rates
(1). Whereas low CSF 5-HIAA concentrations have been linked to a variety of neuropsychiatric disorders
(2), long-term individual stability of CNS serotonin functioning has rarely been investigated in humans, and longitudinal assessments to investigate the genesis of low CSF 5-HIAA concentrations have not been performed in humans, in part because of the difficulty of repeated CSF sampling in human subjects.
Nonhuman primates offer numerous advantages in modeling human psychopathology. Not only are they our closest relatives, but they have a relatively long period of development that, especially in anthropoids, involves considerable dependence on the mother. Furthermore, we can control rearing history in order to test hypotheses concerning the role of early experiences. Consequently, during the past decade, nonhuman primates have been used to assess stability of individual differences in CNS serotonin turnover. Several studies have demonstrated short- and long-term trait-like interindividual stability of CSF 5-HIAA concentrations in nonhuman primate species
(3–
10). Such individual differences are stable across different settings and situations, even in conditions that temporarily increase CSF 5-HIAA concentrations
(3–
6,
8). However, most of the studies have investigated either adult animals or infants who are near the age of weaning
(3,
6,
11–13). Little is known about the earliest periods of development and at what point individual differences in CSF 5-HIAA concentrations stabilize. One study of rhesus macaques, which used a relatively small sample size, reported that neonatal and early infancy CSF 5-HIAA concentrations were not correlated with adult CSF 5-HIAA concentrations and that individual stability did not emerge until after the fourth month of life
(7).
Early rearing history may play a crucial role in the development of the CNS serotonin system of nonhuman primates. Several studies have demonstrated that monkeys removed from their mother at birth and reared in a nursery with age-matched peers (termed peer-reared) exhibit chronically low CSF 5-HIAA concentrations that are evident by late infancy and continue into adolescence and adulthood
(3,
4,
6,
11). However, this finding has not always been replicated
(7,
14), perhaps in part because of methodological differences in the conditions of mother-rearing across studies. Specifically, studies that indicate no differences in CSF 5-HIAA concentrations between peer- and mother-reared animals have utilized relatively small sample sizes and limited the mother-reared rearing condition to mother-rearing in dyads (i.e., mothers and infants housed alone). Mother-only rearing, in the absence of peers, is a form of deprivation rearing, resulting in aberrations in social behavior, unstable dominance relationships, and increased stereotyped behaviors
(15). Therefore, infants reared only with mothers, and thus deprived of peer interaction, may not represent an appropriate control group with which to compare the effects of early nursery rearing.
The primary purposes of the current study were 1) to determine the effects of rearing condition on rhesus monkey CSF 5-HIAA concentrations during early development and 2) to assess the stability of individual differences in those concentrations. In studying the effects of rearing condition, we utilized three conditions: mother-reared in social groups, peer only-reared, and surrogate/peer-reared. Although the effects of early peer only rearing on CSF 5-HIAA concentrations have been well documented, to date no comparable studies have been performed with surrogate/peer-reared infants. Surrogate/peer rearing is widely utilized in nonhuman primate nurseries, and as such, study of the physiological concomitants of this rearing condition is warranted. Furthermore, the surrogate/peer-rearing condition, by combining maternal deprivation with limited peer contact (surrogate/peer-reared infants are provided social contact with other animals only a few hours each day), allows us to further explore those aspects of early experience that may lead to deficits in the serotonin system. In other words, by comparing surrogate/peer-reared monkeys to peer only-reared monkeys—and ultimately to mother-reared monkeys—we can test whether deficits in the serotonin system may result from general social deprivation in addition to maternal absence.
To the degree that individual differences in CSF 5-HIAA concentrations are stable and represent a risk marker for a variety of psychopathologies, establishing how early in life individual differences in CSF 5-HIAA concentrations become stable may provide important clues to assess developmental trajectories and to develop intervention strategies. We therefore designed our study to begin during the neonatal period and to involve repeated sampling of a large number of infants across the first 5 months of life.
Method
Subjects
The subjects were 256 rhesus macaque (Macaca mulatta) infants (144 males and 112 females). At birth, infants were assigned to one of three rearing conditions: mother reared (N=102; 58 males, 44 females), peer reared (N=73; 38 males, 35 females), or surrogate/peer reared (N=81; 48 males, 33 females).
Rearing condition assignments were made at random with the exception that an attempt was made to balance the number of males and females in each rearing condition in a given birth year. Mother-reared infants were housed in social groups approximating natural conditions, with 8–10 adult females (including the infant’s mother), the infant’s father and another adult male, and other similar-aged infants. These social groups were housed in indoor/outdoor enclosures measuring 2.44×3.05×2.21 m (indoor) and 2.44×3.0×2.44 m (outdoor). The floors of the enclosures were covered with wood chips. The lights in the indoor portion of the enclosure were maintained on a 12:12 cycle (7:00 a.m. to 7:00 p.m.). The animals in the social groups were fed high-protein monkey chow and received water ad libitum. Supplemental fruit was provided three times each week; sunflower seeds were presented daily.
Infants assigned to the peer only-reared and surrogate/peer-reared conditions were removed from their mothers between birth and 2 days of age and taken to the neonatal nursery, where they were reared according to standard procedures
(16). During the first 37 days of life, the peer only-reared and surrogate/peer-reared infants were treated identically. From days 1–15 the infants were housed individually in plastic “incubator” cages measuring 51×38×43 cm. Each cage contained an inanimate, rocking “surrogate mother,” composed of a 16.5-cm circumference polypropylene cylinder 25 cm in height attached to an 11.5-cm wide circular metal base by a flexible metal spring. The surrogate mother was covered by an electrical heating pad, which in turn was covered with fleece fabric. A bottle with a nipple was attached to the surrogate mother for purposes of feeding. Loose pieces of fleece covered the floor of the cage. The internal temperature of the plastic “incubator” was maintained at approximately 27°C. The infants could see and hear, but not physically contact, other infants. At 15 days of age, infants were moved into individual wire mesh cages measuring 64×61×76 cm, with their fleece-covered surrogate and fleece blankets. Lights were on a 14:10 hour cycle (7:00 a.m. to 9:00 p.m.). The temperature of the room was maintained between 22°C and 26°C, and the humidity was kept at 50%–55%.
At 37 days of age all nursery infants were placed into the condition (peer only or surrogate/peer reared) randomly assigned at the time of their birth. This took place on day 37 because each subject was run through a novel cage experiment exactly 1 week after day 30, just prior to being placed into a rearing condition. Peer only-reared monkeys were placed in permanent social groups of four age-matched infants, housed together with constant access 24 hours a day in cages measuring 71×81×152 cm. Conversely, surrogate/peer-reared infants continued to be housed individually with their surrogates as described. Surrogate/peer-reared infants were provided with only limited peer contact, being placed in play groups of four individuals, along with their surrogates, in a 71×81×152 cm exercise cage for 2 hours/day, 5 days/week. Following the 2-hour play session, each surrogate/peer-reared infant was returned to its home cage for the remainder of the day. While surrogate/peer-reared infants were provided access to their surrogates at all times throughout the 5 months of the study, infants in the peer only-reared groups retained their surrogates only until the average age of the group members was 4 months.
All peer only-reared and surrogate/peer-reared infants were hand-fed a 50:50 mixture of Similac (Ross Laboratories, Columbus, Ohio) and Primalac (Bio-Serv, Frenchtown, N.J.) formula every 2 hours until 30 days of age. From day 30 until 4 months of age, formula was administered at 400 ml/day, standard high-protein monkey chow was gradually introduced to the diet, and the infants were provided ad libitum access to water. At 4 months the amount of formula provided was reduced to 300 ml/day, and at 5 months it was reduced again to 200 ml/day. At 6 months of age the infants were completely weaned from formula, and provided a diet of monkey chow, supplemental fruit, and water.
Data Collection
Cisternal CSF samples were collected at 14, 30, 60, 90, 120, and 150 days of age. All of the CSF samples were collected in conjunction with blood samples that—on days 14, 30, 90, 120, and 150—were used to measure plasma cortisol and ACTH responses to a moderately challenging condition for a separate study of rearing condition effects on the hypothalamic-pituitary-adrenal (HPA) system. On days 14 and 30, blood and CSF samples were collected following a 30-minute assessment of orientation, temperament, and motor maturity, which involved handling by a human experimenter
(17). On days 90, 120, and 150, subjects were removed from their mother (mother-reared animals), peer group (peer only-reared animals), or home cage (surrogate/peer-reared animals) and isolated in an unfamiliar single cage (64×61×76 cm) for 30 minutes. At the end of the 30-minute period, blood and CSF samples were obtained. On day 60, however, both blood and CSF samples were collected after immediate capture and anesthetization. These samples were intended to provide baseline (nonchallenge) measures of plasma and CSF biochemical measures. All samples were collected between 11:30 a.m. and 2:30 p.m.
For CSF collection, animals were anesthetized using ketamine HCl (intramuscular: 10 mg/kg). One ml of CSF was obtained from the cisterna magna using a 22-gauge needle and 5-cc syringe within 15 minutes of anesthesia. CSF samples were immediately placed in liquid nitrogen and later transferred to a –70° freezer. Samples were assayed for 5-HIAA using high-performance liquid chromatography with electrochemical detection
(18,
19). All intra- and interassay coefficients of variation were <10%.
Data Analysis
The data were first plotted to detect and eliminate possible outliers that could bias the statistical analyses. Preliminary analysis of the data using mixed-design analysis of variance (ANOVA) revealed no significant main effect of sex (F=0.002, df=1, 252, p<0.97) nor any significant interactions between sex and rearing condition (F=2.28, df=1, 252, p<0.14) or sex and age (F=1.85, df=5, 252, p<0.11) on CSF 5-HIAA concentrations. As a result, data for males and females were combined in subsequent analyses. Additionally, comparison of the two groups reared in the neonatal nursery (peer only-reared and surrogate/peer-reared) indicated no differences between these groups in CSF 5-HIAA concentrations (F=0.39, df=1, 152, p<0.54), and similar developmental changes for both groups. Consequently, the peer only-reared and surrogate/peer-reared animals were combined into a single category, nursery reared, for subsequent analyses.
In order to address the two main issues of this study, two types of analyses were conducted. First, we utilized locally weighted scatterplot smoothing to evaluate the effects of early rearing condition (mother reared versus nursery reared) on the development of the serotonin system during the first 5 months of life. Locally weighted scatterplot smoothing fits a nonlinear regression surface to data via local smoothing
(20,
21). A “window,” or a portion of the surrounding data, is used to construct a line or curve at each data point. Locally weighted scatterplot smoothing fits were generated for mother-reared and nursery-reared subjects with the use of macros written for SAS
(22) and modified by Dr. Dennis Young of Arizona State University. First, a range of “windows” (f values) was tested in order to identify the optimal f used to produce the final fits. The optimal f value of 0.2 was identified using the M plot method of Cleveland and Devlin
(21). The final fits for mother-reared and nursery-reared subjects were then compared with the jackknife method proposed by Moses et al.
(23), which addresses the problem of dependent data points that result from repeated measurements on each animal. By using locally weighted scatterplot smoothing, we were able to determine 1) whether CSF 5-HIAA concentrations change over time, 2) whether there was an effect of rearing condition on CSF 5-HIAA concentrations, averaged over time, and 3) whether the pattern of change over time was the same for each rearing condition.
Second, we utilized correlation analyses to assess the stability of individual differences in CSF 5-HIAA concentrations across the first 5 months of life and to determine at what stage in development these individual differences stabilized. Correlation analyses were conducted separately for the two rearing groups (mother reared and nursery reared). Pairwise correlations were calculated across all six time points, resulting in 15 different correlations for each rearing group.
Results
Locally Weighted Scatterplot Smoothing Analysis
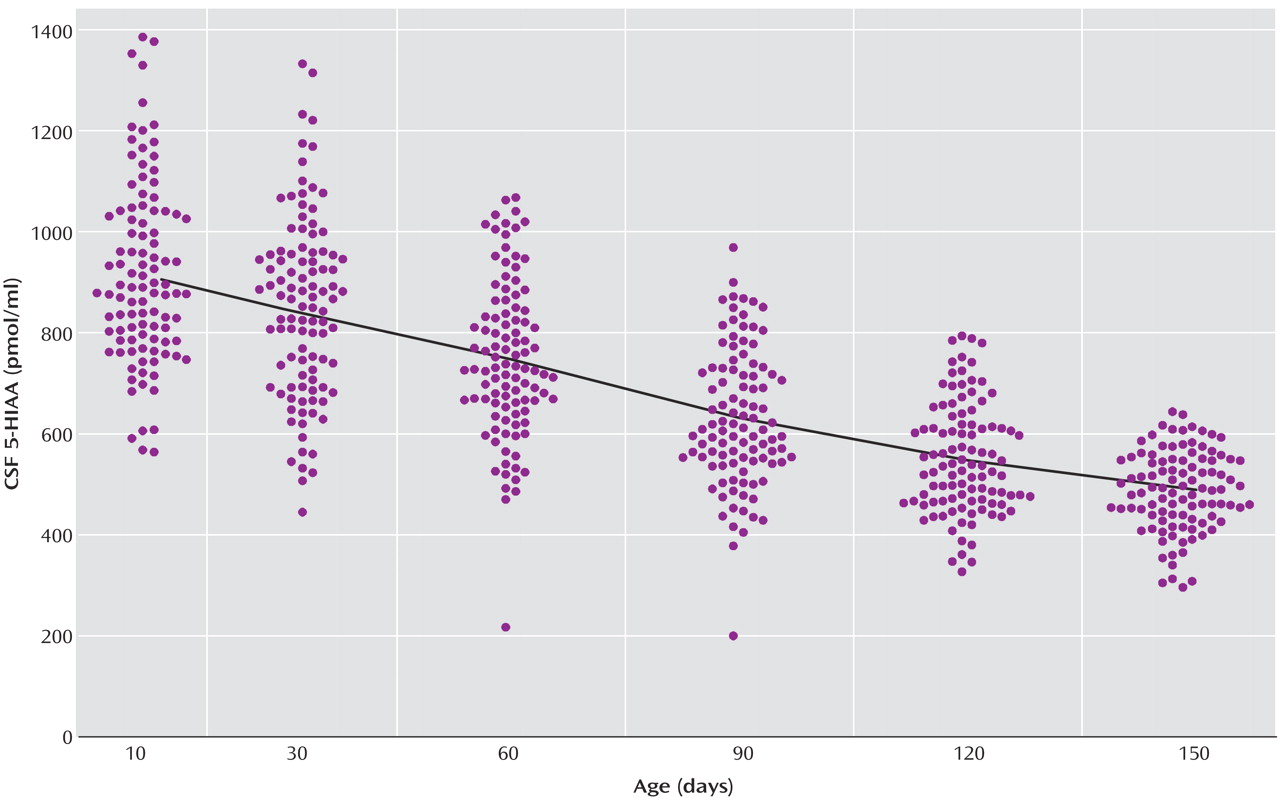
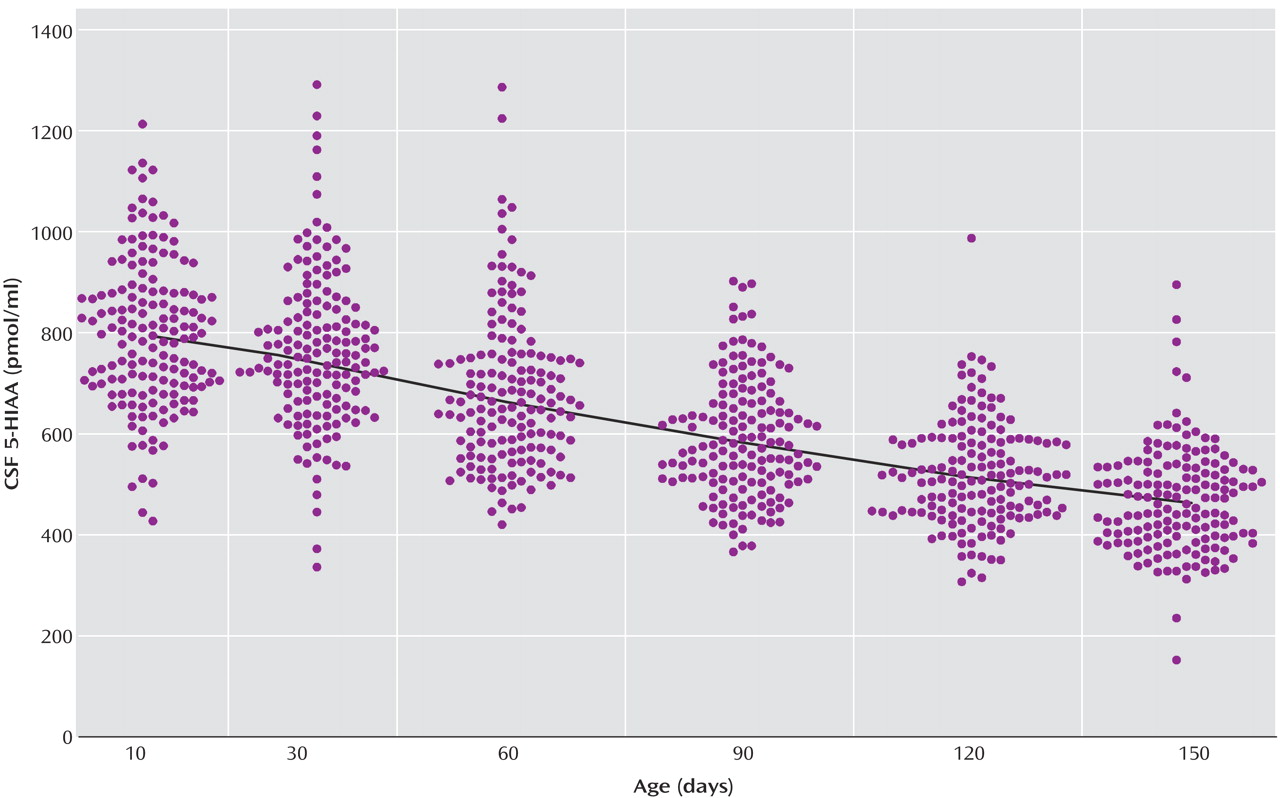
The locally weighted scatterplot smoothing fits for CSF 5-HIAA concentrations in mother-reared and nursery-reared infants during the first 5 months of life are presented in
Figure 1 and
Figure 2, respectively. CSF 5-HIAA concentrations declined throughout this period of early development in both rearing groups. Furthermore, nursery-reared infants consistently displayed lower CSF 5-HIAA concentrations than mother-reared infants (jackknife comparison of locally weighted scatterplot smoothing fits, Z=7.664, p<0.0001). The locally weighted scatterplot smoothing results do suggest that the overall difference between rearing groups is not necessarily consistent across the full length of early development, and that the mother-reared animals become more similar to the nursery-reared animals toward the end of 5 months. However, further analysis of the data indicate significant differences between these two groups at each of the time points (day 14: t=6.02, df=254, p<0.0001; day 30: t=4.30, df=254, p<0.0001; day 60: t=3.62, df=254, p=0.0004; day 90: t=2.97, df=254, p<0.004; day 120: t=2.41, df=254, p<0.02; day 150: t=2.40, df=254, p<0.02).
Correlation Analyses
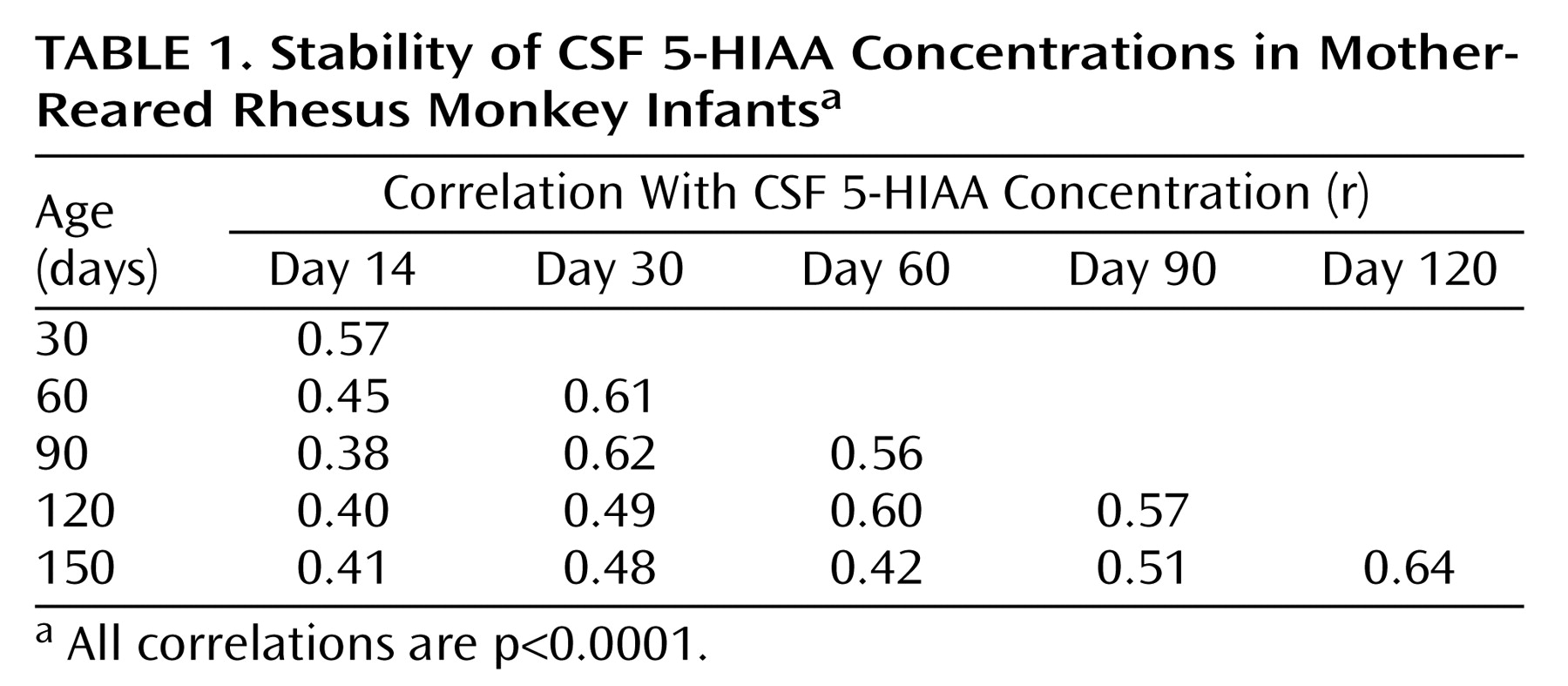
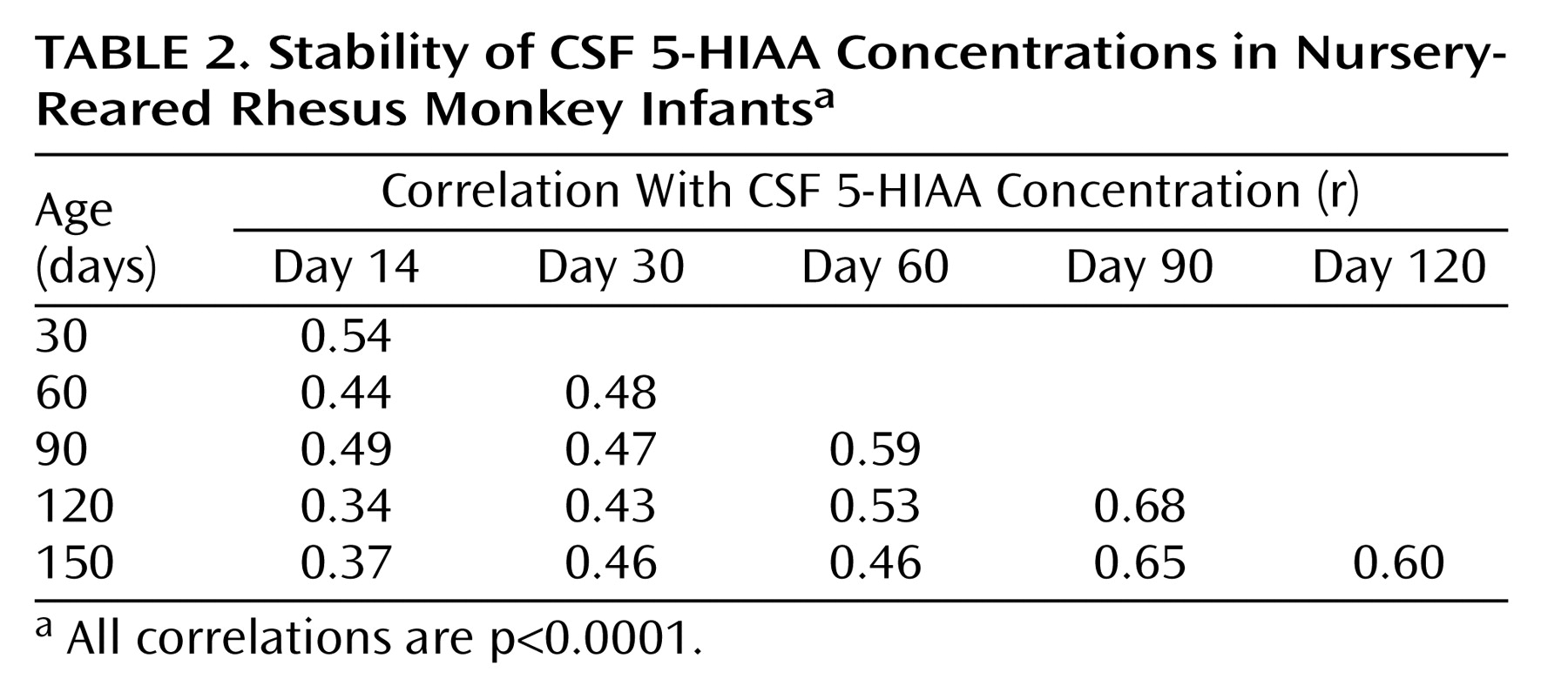
Correlation analyses showed consistent individual stability across each of the measurements in both mother-reared (
Table 1) and nursery-reared (
Table 2) monkeys. In each rearing group, all 15 of the CSF 5-HIAA correlations were positive and statistically significant (p<0.0001), with similar average correlation coefficients seen for mother-reared infants (r=0.51) and nursery-reared infants (r=0.50).
Discussion
Rearing Condition and Early Development
Our results show that rearing condition can affect the early development of the serotonin system in rhesus monkey infants. As early as day 14 of life, nursery-reared infants exhibited lower CSF 5-HIAA concentrations than the mother-reared infants, and they continued to show lower concentrations over the next 5 months. Although some previous studies have failed to find differences in CSF 5-HIAA concentrations between mother-reared and nursery-reared monkeys
(7,
14), the present results indicate that in a large sample of monkeys, nursery-reared infants exhibit lower CSF 5-HIAA concentrations than mother-reared infants when the mother-reared infants were reared in conditions that more closely approximate the species-typical social environment.
Our results also indicate that the quantity of peer interactions in nursery-reared infants did not affect CSF 5-HIAA concentrations. Whether the infants were reared with limited peer contact (surrogate/peer reared) or constant access to other age-mates (peer only reared), their CSF 5-HIAA concentrations were lower than in the mother-reared infants. This, coupled with the lack of significant differences in CSF 5-HIAA between surrogate/peer-reared and peer only-reared monkeys, suggests that maternal deprivation has more of an influence on the developing serotonin system than general social deprivation.
In the nursery environment, the absence of a mother results in a number of important factors being absent or diminished. In the earliest stages of life, these include vestibular stimulation/postural changes provided by mothers’ locomotion, tactile stimulation from handling and grooming, and nutritional provisions from mothers’ breast milk. In a separate study, we found that human handling of nursery-reared infants before day 14 did not result in elevated CSF 5-HIAA concentrations, suggesting that vestibular and tactile stimulation by individuals other than the mother is not necessarily an effective substitute for “mother.” Likewise, commercially available infant formula is not necessarily an effective substitute for a mother’s breast milk, since it is lower in total fatty acid and α-lactalbumin concentrations compared with rhesus monkey and human breast milk
(24–
27). Long-chain essential fatty acids have been linked to healthy development of the brain and central nervous system in human and nonhuman primate infants
(26,
28–34) and may also predict levels of serotonin and dopamine metabolites
(35). The protein α-lactalbumin is rich in tryptophan, a major precursor of CNS 5-HT, and therefore is directly linked to the development of the serotonin system
(27). As nursery-reared infants grow older, there is also a lack of appropriate social feedback. Mothers provide discipline, serve as social referents in situations of uncertainty, and act as models for the development of appropriate social behaviors. It is unlikely that nursery-reared infants receive the same benefits from their interaction with same-aged peers.
Physical differences between the nursery- and mother-rearing environment, such as light cycles, cage size, and physical and social complexity of the environment, may also play a role in early developmental differences between mother-reared and nursery-reared monkeys. However, the fact that rearing condition differences in CSF 5-HIAA concentrations were found as early as day 14 suggests a limited role, if any. During the first 2 weeks of life, infants are very rarely found off their mothers’ ventrums, and as such their experience of the wider environment is limited. This suggests that the differences in serotonin function observed in nursery-reared and mother-reared infants at such an early age are more likely being driven by factors related specifically to the mother and the microenvironment she provides. These mother-specific factors likely contribute to the continuing differences observed between mother-reared and nursery-reared monkeys over the next months of life.
The developmental decline in CSF 5-HIAA levels that we observed during the first 5 months in both nursery-reared and mother-reared infants is consistent with other studies of nonhuman primates as well as with studies of human children of comparable developmental ages
(3,
6,
7,
36–40). However, while these studies report declines in CSF 5-HIAA concentrations from infancy into late adolescence or young adulthood, the current study shows that concentrations actually begin to decline as early as day 30. To our knowledge, this is the first comprehensive report of a developmental decline in the early infancy period of either humans or nonhuman primates.
Stability of Individual Differences
Despite developmental declines in absolute 5-HIAA concentrations, individual differences in CSF 5-HIAA exhibited trait-like stability across repeated measurements, as exemplified by high positive correlation coefficients across repeated measurements for both mother-reared and nursery-reared infants. These results are consistent with previous findings concerning juvenile and adult nonhuman primates
(3–
10). Such stability of individual differences may be present in humans as well, although results have been conflicting
(36,
41).
As stated earlier, CSF samples were collected under both moderate challenge conditions (days 14, 30, 90, 120, and 150) and a “baseline” condition (day 60). The moderate challenge conditions are known activators of the HPA axis in all rearing groups
(42). Because the monoamines are stress-sensitive, it is possible that the 5-HIAA measures taken under the moderate challenge conditions reflected increased CNS serotonin activity. However, we were unable to test this possibility. First, due to the necessity of anesthetizing animals for sample collection, it was impossible to collect both baseline and postchallenge samples on the same day, which would have allowed us to directly compare these values. Second, due to the developmental decline in CSF 5-HIAA concentrations, a direct comparison of the day 60 baseline samples and the postchallenge samples obtained on the other days would have been inappropriate. We do note that across both baseline (day 60) and challenge conditions (days 14, 30, 90, 120, and 150), the developmental decline is still evident, and individual differences in CSF 5-HIAA concentrations remained relatively stable, despite the differences in sampling technique.
Serotonin, CNS Development, and Psychopathology
CSF 5-HIAA is thought to measure CNS serotonin functioning by measuring aggregate CNS serotonin turnover
(1). While specific neuronal responses cannot be pinpointed using this method, a number of studies have found that CSF 5-HIAA concentrations are correlated with serotonin turnover in the frontal cortex
(1,
43–45). Relative to the entire lifetime of the rhesus monkey, major developmental processes are taking place in the cortical areas of the brain, including the frontal cortex
(46), during the first 6 months of life. Psychological processes such as emotional self-regulation and impulse control first emerge during this same period, in parallel with CNS development. From an evolutionary perspective, it is clear that for normative development of the CNS system to occur, environmental input is of critical importance, especially during such sensitive periods
(47). As such, evolution has selected for mothers to provide emotional security and to punish inappropriate social impulses, perhaps providing the input needed to properly link the serotonin system to the frontal cortex region, which is of critical importance in both emotional regulation and impulse control. While speculative, it is possible that with the absence of a mother to provide appropriate input, offspring may be at risk for serotonin-mediated affective disorders and impulse control deficits.
Our results have important ramifications for understanding the repetitive nature of psychopathology and relapse. Temperament and personality are widely recognized as contributing to a predictable style of social interaction as well as to axis II psychopathology. To the degree that psychopathology is influenced by the serotonin system, our findings suggest that the risk for psychopathology and serotonin-mediated axis II personality disorders may be exacerbated by experiences early in life that impair the function of the central serotonin system.
In conclusion, this study demonstrates that individual differences in CSF 5-HIAA concentrations are stable and trait-like across the neonatal period in rhesus macaques. These results suggest that low CSF 5-HIAA concentrations are an enduring, stable biological marker that may have roots in early infancy. Furthermore, our findings show that early nursery rearing reduces CNS serotonin turnover, possibly increasing the risk for serotonin-mediated psychopathologies.