Although the last five decades have seen many pharmacological advances in the treatment of depressive illness, still only half of all patients respond to their initial antidepressant treatment, leaving the remaining half symptomatic and functionally impaired
(1). There is little empirical basis to guide clinicians in selecting one medication over another. The potential benefits of discovering predictors for individual antidepressants are considerable because patients could be matched to a medication to which they are most likely to respond. Because the first choice of treatment for the majority of depressed patients is a selective serotonin reuptake inhibitor (SSRI), it would also be useful to prospectively identify patients who are likely to be unresponsive to SSRI monotherapy because they could be given some alternative.
Presumably, differences in underlying pathophysiology account for the variability in antidepressant treatment response. To the extent that biological heterogeneity is reflected in symptom heterogeneity, phenomenology should inform treatment selection. Psychomotor retardation, a symptom exhibited by a subgroup of patients with depression, has been empirically linked with a specific pathophysiology and may therefore be a candidate predictor of antidepressant treatment outcome. Several lines of evidence link an underlying dopaminergic abnormality to the expression of psychomotor retardation, defined as a general slowing of motor activity and difficulty responding spontaneously and quickly to the environment. First, because psychomotor retardation is comparable to bradykinesia (i.e., a slowing down of spontaneous movement) and bradyphrenia (i.e., a slowness in thinking) in Parkinson’s disease, it has been suggested that, like patients with Parkinson’s disease, depressed patients with psychomotor retardation may have an abnormality in dopaminergic functioning. Moreover, decreased levels of CSF homovanillic acid, the main metabolite of dopamine, has been reported in depressed patients with psychomotor retardation
(2,
3), and more recently, brain imaging techniques using dopamine D
2 receptor binding have demonstrated reduced dopaminergic striatal functioning in the caudate-putamen of depressed patients with psychomotor retardation
(4,
5).
The speed of psychomotor processing, a quantitative measure of psychomotor retardation assessed with neuropsychological tests (e.g., reaction time, speech rate, motor speed, mental speed, initiation and spontaneity of response), has also been linked to dopaminergic functioning. For example, reduced verbal fluency and poor performance on the digit symbol substitution test of the WAIS have been correlated with decreased dopamine functioning in the striatum
(6). The influence of striatal dopamine on verbal fluency is supported by the finding that patients with Parkinson’s disease who discontinued levodopa exhibited impaired verbal fluency, which significantly improved when they resumed taking levodopa
(7). Together, these studies suggest that verbal fluency and psychomotor speed may be contingent upon brain dopamine levels in the striatum.
It is reasonable to assume that in addition to dysfunction of dopaminergic striatal areas, an abnormality at any level within the functional network involved in psychomotor retardation would also result in psychomotor slowing (e.g., in regions within the dorsolateral prefrontal circuit that connect to the striatum). This is supported by the consistently replicated finding of an inverse correlation between psychomotor retardation and blood flow or metabolism to the dorsolateral prefrontal cortex of the left hemisphere
(8,
9) and bilaterally
(10,
11). Although reduced dorsolateral prefrontal perfusion has also been reported in depressed patients as a whole (i.e., with or without psychomotor retardation)
(12,
13), as well as in other patient populations, such as those with schizophrenia
(14,
15), it has been suggested that this is a result of subgroups, each with psychomotor slowing, in the groups studied. For example, the use of reduced speech to quantify psychomotor speed in depressed and schizophrenic patients with reduced speech output showed decreased blood flow in the left dorsolateral prefrontal cortex that was evident even after control was added for dysphoria and diagnoses
(8).
Taken together, various lines of investigation suggest an association between processing speed and dopamine levels and a link between psychomotor retardation and abnormal functioning of the basal ganglia and the dorsolateral prefrontal cortex. This suggests that depressed patients with psychomotor slowing may have a dopaminergic abnormality within frontal-subcortical networks that causes or contributes to the pathophysiology of their disorder. This may have important treatment implications because these patients may preferentially benefit from a medication that directly targets dopaminergic neurotransmission. As such, one might predict that these patients would be unresponsive to SSRI antidepressants that primarily enhance serotonergic functioning. The only study to our knowledge that has investigated whether psychomotor speed on neuropsychological tests is related to SSRI response in depressed adults found no association
(16). Of importance, however, the small group of 14 patients in this study limited its power to detect group differences. The current study investigated whether psychomotor speed would predict response to 12 weeks of SSRI treatment in a group of moderately depressed adult outpatients. We hypothesized that psychomotor slowing, measured with neuropsychological tests, would predict nonresponse to fluoxetine.
Method
The subjects were recruited from an outpatient research clinic at the New York State Psychiatric Institute. All patients met DSM-IV criteria for major depressive disorder and were between ages 18 and 65 years. Subjects were excluded if they met DSM-IV criteria for delusional, psychotic, bipolar, antisocial, personality, substance use, or organic mental disorders; schizophrenia; or the presence of psychotic features. The subjects did not have unstable physical disorders, acquired brain injury, degenerative diseases, cognitive changes following medical illness or surgery, memory disorders, language disorders, learning disability, or seizure disorder. All patients were native English speakers. After complete description of the study to the subjects, written informed consent was obtained.
The study design was a 12-week open trial of fluoxetine treatment with a 7–10-day medication-free lead-in period. During the lead-in period, the subjects were administered a short battery of neuropsychological tests. After the lead-in period, the patients whose depression was rated “much improved” or “very much improved” from baseline severity according to the Clinical Global Impression (CGI) Scale did not enter the treatment phase of the study. The patients whose depression was rated “minimally improved” to “worse” began a fixed flexible-dosing trial with fluoxetine (10 mg/day at week 1, 10–20 mg/day at weeks 2–4, 10–40 mg/day at weeks 5–8, and 10–60 mg/day at weeks 8–12). Response at week 12 was rated by an independent evaluator who was blind to the patients’ neuropsychological test results and the course of response or nonresponse during the 12 weeks. The patients who no longer met the criteria for major depression and had a CGI Scale score of “much improved” or “very much improved” were considered to be fluoxetine responders. All others were nonresponders.
Forty-seven patients who completed the neuropsychological test battery entered into the acute treatment phase. Ten dropped out before the end of the study, resulting in a total of 37 patients who completed a 12-week trial with fluoxetine. There were no differences between the dropouts and the completers on any neuropsychological or clinical measures.
Table 1 gives the demographic and clinical characteristics of the 25 patients who were rated as fluoxetine responders and the 12 patients who were classified as nonresponders. There were no significant differences between the groups in gender, age, educational level, age at onset of the first depressive episode, pretreatment depression severity, or estimated pre-illness cognitive ability (vocabulary subtest of the WAIS-III).
The neuropsychological tests were selected based on prior research that associated psychomotor speed with dopaminergic functioning, with brain areas within striatofrontal circuitry, and/or with antidepressant response. Since performance on these tests requires the recruitment of many cognitive abilities, some control measures were administered to evaluate the specificity of the function in question.
The overall difference on neuropsychological measures of psychomotor speed was assessed through a multivariate analysis of variance (MANOVA) with performance on the four tests as the dependent measure and treatment response as the between-subject factor. Follow-up univariate t tests were then conducted.
The total 17-item Hamilton Depression Rating Scale (HAM-D) score at week 12 (i.e., depression severity at the end of the study) was used as an alternative means of quantifying response to fluoxetine treatment. Backward multiple regressions were used to predict HAM-D score outcome with measures of psychomotor speed as predictor variables. Pearson’s product-moment correlation analysis examined the relationship between performance on neuropsychological measures and pretreatment clinical variables.
A secondary analysis with independent t tests was employed to examine performance differences between responders and nonresponders on tasks measuring function in other cognitive domains, including attention, executive functioning, visuospatial functioning, and verbal intelligence.
Results
MANOVA indicated that the overall difference on neuropsychological measures of psychomotor speed significantly differentiated fluoxetine responders from nonresponders (Wilks’s lambda=0.639, F=4.51, df=4, 32, p=0.005). The results of follow-up univariate t tests are summarized in
Table 2. At baseline, the patients who subsequently did not respond to fluoxetine verbalized significantly fewer words on the Controlled Oral Word Association Test FAS (COWAT FAS)
(17) and named significantly fewer colors on the Stroop Color and Word Test
(18) compared to the responders. Although the results were not statistically significant, the nonresponders tended to perform worse than the responders on the remaining two measures of processing speed (i.e., the Stroop Color and Word Test word reading subtest and the WAIS-III digit symbol subtest). Effect sizes for all four measures ranged from medium to large (0.61–1.44).
A backward multiple regression analysis with control for baseline depression severity demonstrated that of the tests of psychomotor speed, the baseline COWAT FAS significantly predicted week-12 HAM-D scores, with the model explaining 33% of the variance (
Table 3). This, together with the significant inverse correlation between the COWAT FAS scores and the week-12 HAM-D scores (r=–0.535, p=0.001) and the weaker associations between the HAM-D scores and those of the other timed tests (e.g., the Stroop Color and Word Test color naming subtest: r=–0.256, p=0.13; the Stroop Color and Word Test word reading subtest: r=–0.240, p=0.15; and the WAIS-III digit symbol subtest: r=–0.182, p=0.28), suggests that COWAT FAS performance may be the strongest predictor of fluoxetine response.
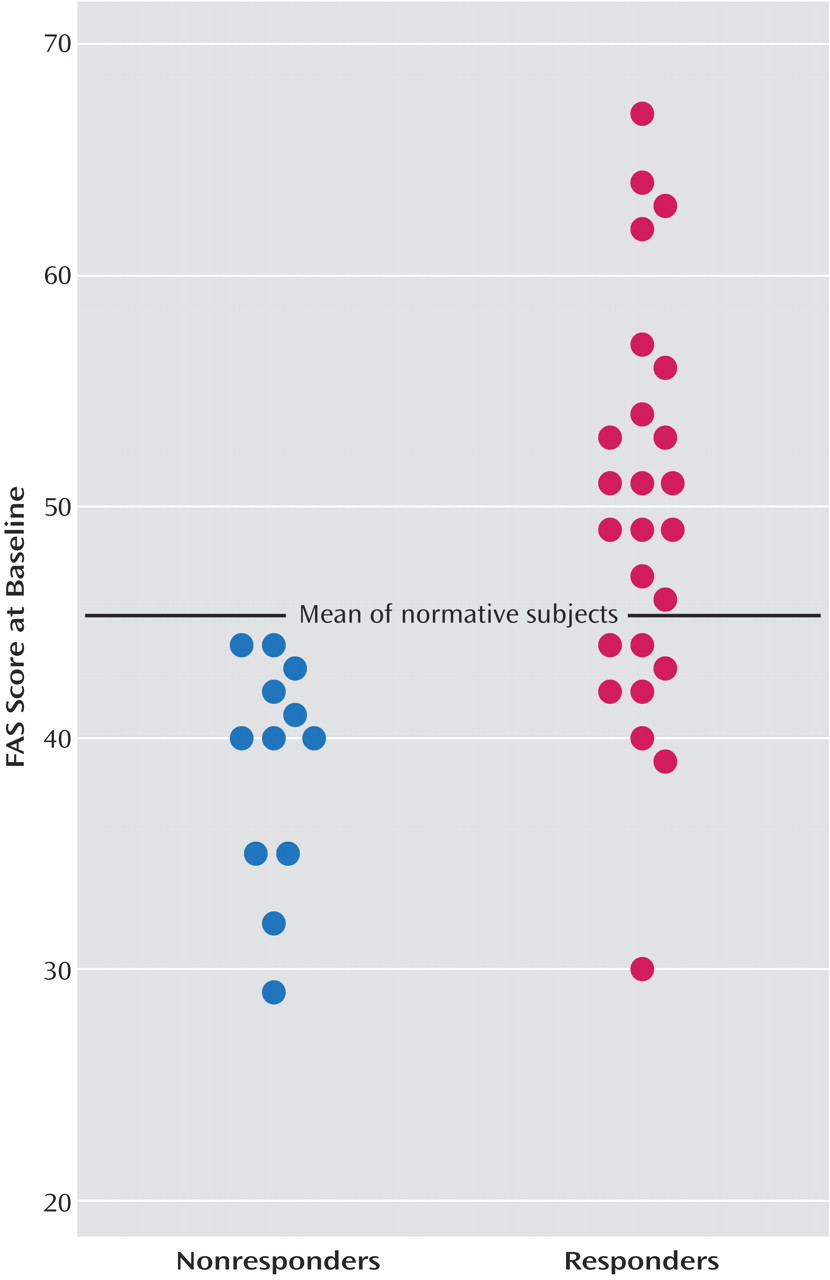
Figure 1 plots the individual COWAT FAS scores at baseline for the fluoxetine responders and the nonresponders, together with the published COWAT FAS normative scores for a healthy group matched to the depressed patients by age and level of education
(19). As shown, all of the nonresponders generated fewer words at baseline than the normative mean, with the difference between the two groups approaching significance (t=–1.83, df=252, p<0.07). The depressed patients who responded to fluoxetine performed significantly better on the COWAT FAS than the normative group (t=2.22, df=265, p<0.03). With the mean for the normative group used as a cutoff, those who scored below the mean had a response rate of only 40% (eight of 20) to fluoxetine. In contrast, 100% (17 of 17) of the depressed patients who scored above the mean responded to fluoxetine.
No significant relationships were found between processing speed and pretreatment depression severity, chronicity, or age at onset of the first depressive episode (range=–0.213 to 0.105). A backward multiple regression analysis predicting outcome with control for these baseline characteristics again demonstrated that of the measures of processing speed, only the COWAT FAS scores significantly predicted the outcome of HAM-D scores.
The differentiation of responders and nonresponders according to baseline physician-rated psychomotor retardation (i.e., the HAM-D psychomotor retardation item) approached significance; 50% of the nonresponders (six of 12) were judged to have psychomotor retardation at baseline versus 16% of the responders (four of 25) (χ2=3.19, df=1, p<0.08). To assess whether physician-rated psychomotor retardation is as powerful a predictor of outcome as the four measures of psychomotor speed, a backward multiple regression analysis was performed. After we controlled for baseline severity (HAM-D scores minus the psychomotor retardation item scores), only the COWAT FAS (β=–0.281, SE=0.093, p=0.005) and the HAM-D psychomotor retardation item (β=3.352, SE=1.878, p<0.09) remained in the model. Baseline depression severity did not significantly predict outcome.
In terms of performance in other cognitive domains, no significant differences were found between the responders and the nonresponders on the Stroop Color and Word Test interference score, the Wisconsin Card Sorting Test (number of categories completed and number of perseverative errors)
(20), or the WAIS-III subtests for block design, digit span, or vocabulary (
Table 2).
Discussion
This study was designed to investigate whether baseline performance on neuropsychological tests of processing speed predicts response to fluoxetine in depressed outpatients. The patients who were resistant to 12 weeks of fluoxetine treatment exhibited significantly reduced pretreatment performance on the COWAT FAS of verbal fluency and the Stroop Color and Word Test color naming subtest compared to the patients who responded to fluoxetine. A less-than-significant tendency in the same direction was demonstrated for the Stroop Color and Word Test word reading subtest and the WAIS-III digit symbol subtest.
If, as previously discussed, depressed subjects with psychomotor slowing are unresponsive to the serotonin-acting agent fluoxetine because of a neural dopaminergic dysfunction, one would expect that treatment that directly targeted dopamine neurotransmission might be effective. This was nicely demonstrated by Rampello et al.
(21), who assessed the effectiveness of antidepressants with different affinities to dopamine in patients diagnosed with retarded depression. After 6 weeks of treatment, the patients who were treated with amineptine (a selective inhibitor of dopamine reuptake) showed greater improvement on both the Psychomotor Retardation Scale and the HAM-D than the patients treated with minaprine (a nonselective reuptake inhibitor), clomipramine (affects serotonin and, to a lesser extent, norepinephrine), and placebo. Furthermore, the patients had a better response to minaprine than to clomipramine, and clomipramine response was not superior to that of placebo. This suggests that in depressed patients with psychomotor retardation, there is a graded response to treatments with increasing affinities to dopamine. The poor response in patients with psychomotor retardation to clomipramine, a drug that primarily affects serotonin, lends validity to the current findings of an unfavorable response to an SSRI in patients with psychomotor slowing.
Of interest, verbal fluency and psychomotor retardation have also been associated with treatment outcome in patients with late-life depression. In particular, Kalayam and Alexopoulos
(22) found that elderly depressed patients who were unresponsive to antidepressants (the majority of which were SSRIs and tricyclics) showed baseline psychomotor retardation, a prolonged latency of the P300 evoked potential (an electrophysiological measure of psychomotor speed), and a low initiation-perseveration score (which consists largely of verbal fluency) on the Mattis Dementia Rating Scale compared to responders. The present study identified a younger subgroup of depressed patients also characterized by reduced verbal fluency, psychomotor retardation, and SSRI resistance, suggesting a possible common underlying pathophysiology between the younger and geriatric subgroups.
The findings of this study were specific to tests of processing speed. Fluoxetine responders and nonresponders demonstrated similar performance on tasks that index functioning in other cognitive domains, including executive functioning, attention, visuospatial functioning, and verbal intelligence. This suggests not only that processing speed uniquely predicted fluoxetine response but also that these other cognitive functions had a negligible contribution to the nonresponders’ reduced processing speed. For example, since fluoxetine responders and nonresponders performed similarly on a test of attention, attentional differences between the groups could not account for the differences exhibited on the timed tasks. The specificity of a differential treatment response based on measures of psychomotor speed is supported by the study of Kalayam and Alexopoulos
(22) in which antidepressant responders and nonresponders differed in baseline psychomotor slowing but not on tasks of attention, conceptualization, memory, or construction.
The COWAT FAS emerged as the strongest predictor of fluoxetine response, followed by clinician ratings of psychomotor retardation. Because this test measures the initiation and speed of verbal response, clinicians may have detected and rated slowed or reduced verbal processing at baseline as psychomotor retardation in fluoxetine nonresponders. That the COWAT FAS is a more powerful predictor than clinicians’ ratings, however, suggests that it is more sensitive. Nonetheless, the contribution of reduced verbal processing to psychomotor slowing in fluoxetine nonresponders is consistent with previous findings by our group. In the studies that used dichotic measures to assess hemispheric laterality, fluoxetine nonresponders demonstrated a reduced advantage of the left hemisphere for verbal processing compared to responders
(23–
25). Conversely, the responders had a significantly larger left-hemisphere advantage for verbal processing than both the nonresponders and the normal comparison subjects. Similarly, in the present study, verbal processing measured with the COWAT FAS was lower for the nonresponders than that of the published norms matched for age and education, whereas the mean performance of the responders was significantly higher than the normative mean. Neuroanatomically, because the left dorsolateral prefrontal cortex and the left anterior cingulate are activated during verbal fluency tasks
(26–
29), activation of these brain structures may differ between fluoxetine responders and nonresponders. This is supported by neuroimaging studies that found increased baseline rostral anterior cingulate and dorsolateral prefrontal cortical activity in depressed patients who subsequently responded to antidepressant treatment
(30–
33).
This study has several limitations. First, it was an open treatment study (i.e., with no placebo control group), and therefore, it is unknown whether response was attributable to the drug or to something nonspecific. Another drawback is that the predictive value of the findings does not necessarily generalize to SSRIs other than fluoxetine, and therefore, response to various SSRIs, in addition to other classes of antidepressants, should be examined in patients with slow processing speed. Finally, because the flexible-dose design used in this study may have led to an underestimation of the predictive value of psychomotor slowing, it would be worthwhile for future studies to use a fixed dose.
Psychomotor speed as a predictor of fluoxetine response has important clinical and heuristic implications. On a clinical level, prospectively identifying patients who are unlikely to respond to one of the most frequently prescribed antidepressants would guide physicians to initiate treatment with an alternative medication. This is critical because it can potentially reduce the time to symptom relief and increase treatment compliance. Furthermore, it can prevent the premature discontinuation of treatment that often results from feelings of hopelessness, helplessness, and frustration due to an ineffective therapeutic response. Another significant advantage of using cognitive measures to predict treatment response is that unlike some other approaches that may eventually offer predictive value (e.g., brain imaging), tests of psychomotor speed are noninvasive, are of minimal cost, and can be performed quickly. For example, the COWAT FAS can be administered in 5 minutes. On a heuristic level, depressed patients with psychomotor retardation may define a homogeneous subgroup of depressed patients with a unique pathophysiology and prognosis for different classes of antidepressants. Studying this homogeneous entity with functional neuroimaging will help to elucidate the functional neural networks involved in its underlying pathophysiology and possibly reveal structural and functional differences between fluoxetine responders and nonresponders.