Chemokines are proinflammatory cytokines with the ability to attract and activate leukocytes. They also modulate the functions of numerous other cell types. For example, chemokines and their receptors are implicated in the regulation of a variety of normal functions in the brain, including those of neurodevelopment, intercellular communication, and neuronal survival
(1). Accordingly, abnormalities in chemokine functions may predispose for schizophrenia and other psychiatric illnesses. In support of this, psychiatric changes such as psychotic schizophrenia-like manifestations have been observed in humans upon treatment with cytokines
(2). Moreover, some studies have revealed abnormalities in the secretion and blood levels of cytokines in schizophrenia patients
(3,
4).
Disturbances in chemokine levels can be induced by infectious agents. In prenatal life or early childhood, this might have a profound effect on the development of some brain functions
(2). In fact, the increased risk for schizophrenia in adulthood associated with prenatal exposure to common viruses could be a consequence of cytokine disturbances induced by these viruses
(5,
6). A retroviral etiology of schizophrenia has also been suggested
(5). Recent observations have provided additional support to this hypothesis by identification of retroviral transcripts in CSF from schizophrenia patients
(7).
The chemokine receptor CCR5 binds a number of chemokines and is also the major coreceptor for the macrophage-infecting strains of HIV
(8). It is expressed on various WBC subsets as well as nonhematopoietic cells
(9). In the brain, the expression of CCR5 varies with developmental stage, cell type, and brain region
(1). A common nonfunctional allele of the CCR5 gene characterized by deletion of a 32-bp segment in the open reading frame confers resistance to infections with macrophage-infecting strains of HIV
(10,
11). This allele has also been associated with susceptibility to a variety of other immune-related diseases, including diseases of presumed autoimmune etiology
(12–
15). In addition, there is evidence that it affects the severity and course of this group of diseases
(16–
18).
Schizophrenia is probably a disease with a significant degree of etiological heterogeneity. Several clinical subsets of this disease have been described, including a late-onset form. Although the existence of such a schizophrenia subset is controversial, there does seem to be sufficient evidence to define schizophrenia with onset after 40 years as a distinct clinical entity
(19,
20).
A variety of environmental factors have been associated with onset of schizophrenia, but their importance seems to be limited in comparison with the genetic contribution
(21). Previous observations have suggested that genetic or prenatal factors determine age at onset in schizophrenia rather than environmental factors in postnatal life
(22).
This study tests two hypotheses. The first of these hypotheses proposes that the CCR5 deletion allele affects fundamental neurodevelopmental processes and that interactions with prenatal viral infections cause developmental alterations in important brain circuits predisposing for schizophrenia in adulthood. The second hypothesis proposes that these neurodevelopmental alterations affect the age at which first schizophrenia symptoms appear, consistent with previous observations suggesting that genetic or prenatal factors are more important determinants of age at onset of schizophrenia than postnatal factors
(22).
Method
Subjects
Included in the study were 268 patients (109 women and 159 men, female/male ratio=0.69) fulfilling ICD-10 schizophrenia criteria (F20). The age of the patients ranged from 18 to 85 years with a median of 41.0 years. They were recruited from Denmark psychiatric hospital departments in the greater Copenhagen area. High diagnostic reliability was confirmed on a random subset of patients with the OPCRIT instrument
(23). All patients as well as their parents were born in Denmark. Information on age at first admission to a psychiatric hospital department was obtained from medical records and hospital discharge registers. This age was used as a measure of the age at onset of schizophrenia. The age at first admission of most of the patients ranged from 20 to 30 years. About 19% had an age at first admission above 40 years. A total of 67 patients were interviewed and asked about their age at appearance of first symptoms. This age was strongly correlated with age at first admission (correlation coefficient=0.52, p<0.0000065). Among female patients, age at first admission ranged from 10 to 63 years with a median of 29.0 years. Among male patients, the median age at first admission was slightly lower (28 years, range=10–67). Eight of the patients (four of each gender) represented cases of early onset or very early onset schizophrenia, having been admitted to psychiatric hospital departments before reaching 18 years of age
(24). Late-onset schizophrenia was defined as a disease entity with onset after 40 years of age
(19).
Also included in the study were 323 anonymous blood donors (136 women, 187 men, female/male ratio=0.73) serving as healthy comparison subjects. They were all Caucasians recruited from the same geographic area as the patients. Their ages ranged from 20 to 67 years with a median of 42 years.
The study was approved by the Danish Scientific-Ethical Committees and the Danish Data Protection Agency. A majority of the patients and some of the comparison subjects had also been included in a previous study of the genetic susceptibility to schizophrenia
(25). All patients had given written informed consent prior to participation in the study. At the time of recruitment, no subject was subdued with civil or forensic psychiatric restraints.
Genotyping
Genotyping of the CCR5 32-nucleotide deletion polymorphism was accomplished by enzymatic amplification of genomic DNA and agarose gel electrophoresis of amplified fragments
(26). Genomic DNA was derived from peripheral blood.
Statistical Analyses
Agreement of observed genotype proportions with those expected under conditions of Hardy-Weinberg equilibrium was assessed by chi-square test. Genotype and allele distributions were compared by using chi-square test and Fisher’s exact test. The Mann-Whitney test and the Kruskal-Wallis test were used to compare distributions of age at first admission in different genotype categories. Results yielding a p value <0.05 were considered statistically significant.
Results
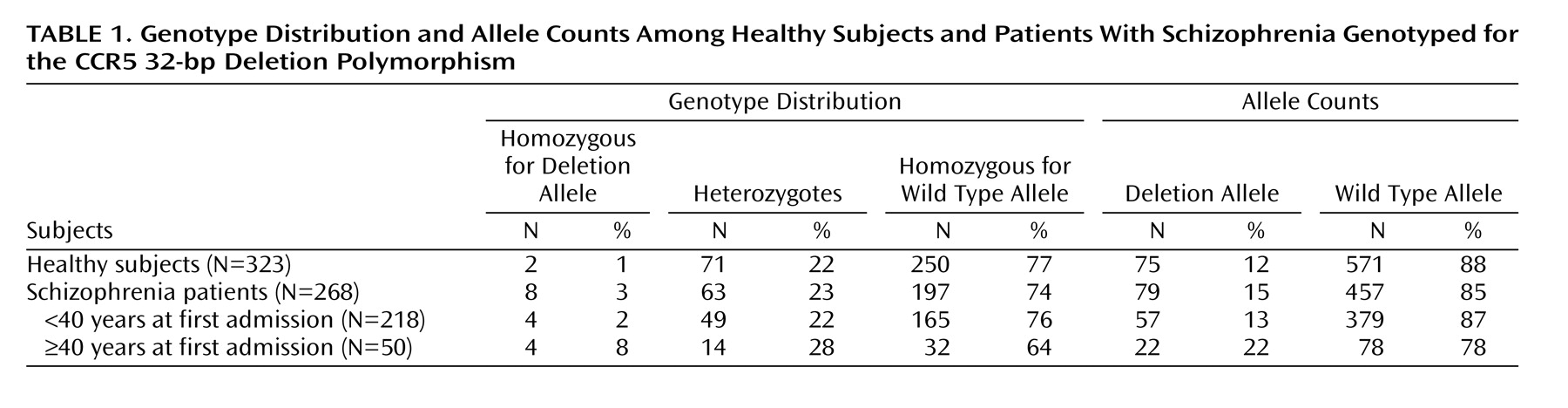
The genotype proportions did not deviate significantly from those expected under conditions of Hardy-Weinberg equilibrium among healthy subjects (χ2=1.63, df=1, p=0.20) or patients (χ2=1.12, df=1, p=0.29). Neither did the genotype distributions of patients with age at first admission below or above 40 years of age deviate significantly from Hardy-Weinberg expectations.
Genotype and allele distributions are shown in
Table 1. The distributions of genotypes in the group of patients differed marginally from that of the comparison subjects (χ
2=5.29, df=2, p=0.07), essentially due to a higher number of the genotype homozygous for the deletion allele in the patient group. Analysis of the allele distribution of these two groups failed to reveal a significant difference (p=0.12, Fisher’s exact test).
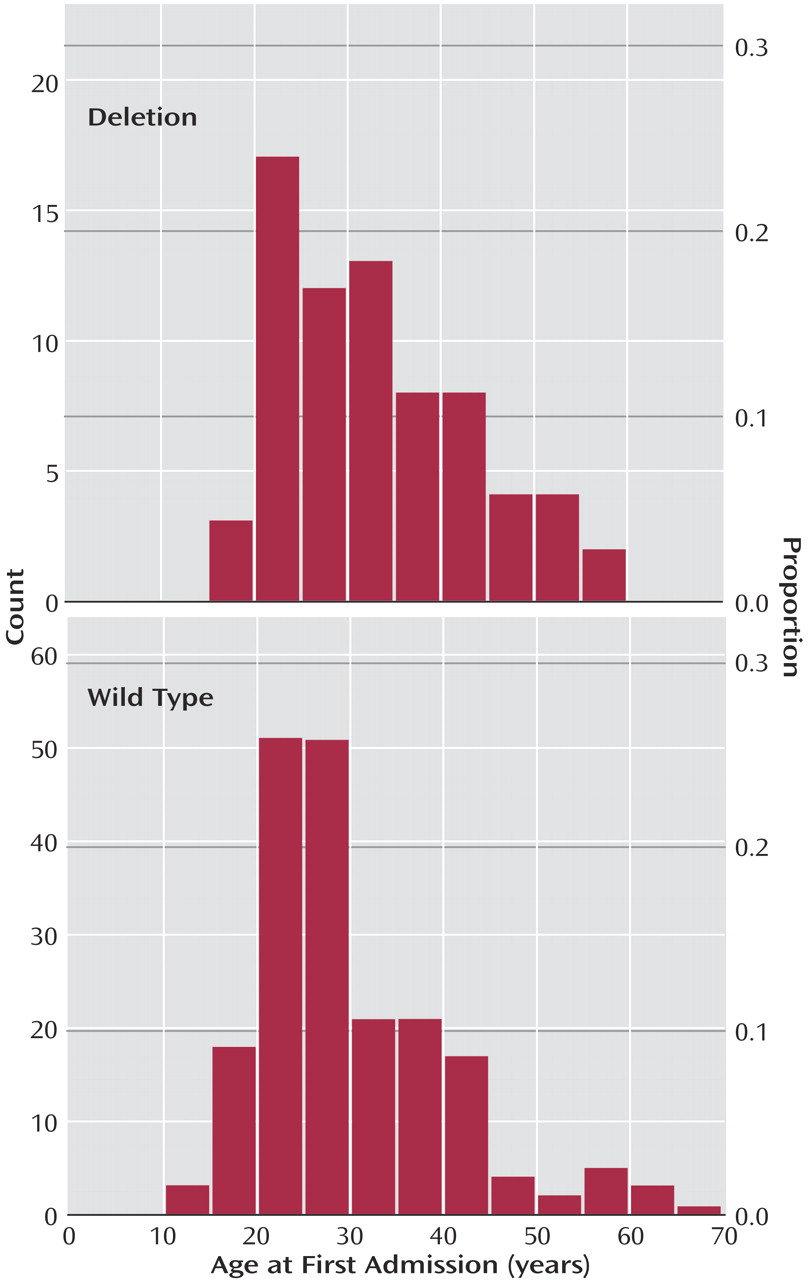
We analyzed the age at first admission among the schizophrenia patients and found that the distribution of this age differed marginally between the three categories of CCR5 genotypes (chi-square approximated test statistic=5.95, df=2, p=0.05) with a higher median among patients homozygous for the deletion allele (37 years) than heterozygotes (31 years) and wild type allele homozygotes (27 years). Collapsing of the genotypes heterozygous and homozygous for the 32-bp deletion allele (median of collapsed group=32 years) provided further evidence in support of a relationship between this allele and age at first admission (chi-square approximated test statistic=5.75, df=1, p<0.02) (
Figure 1). To assess whether possible changes in the procedure of patient registration over time could have influenced our results, we excluded patients born before 1950. On analysis of the remainder (N=222), we still found deletion allele carrier status to be associated with higher ages at first admission (chi-square approximated test statistic=4.61, df=1, p=0.03).
Subsequently, we dichotomized the age at first admission using 40 years as the cutoff and considered schizophrenia with an age at first admission of 40 years and older as a distinct disease entity (
Table 1). Using this approach we did not find that the genotype distributions differed significantly between healthy subjects and patients with ages at first admission below 40 years (χ
2=1.80, df=2, p=0.41); neither was there evidence that the allele distributions differed (p=0.51, Fisher’s exact test). In contrast, the genotype distributions of healthy subjects and patients with an age at first admission of 40 years and older differed significantly, in particular due to a higher frequency of the genotype homozygous for the deletion allele in the latter group (χ
2=16.38, df=2, p=0.0002). The allele distributions also differed significantly between these two groups (p=0.006, Fisher’s exact test), reflecting a higher frequency of the deletion allele in the patient subgroup. On comparison of the two patient subgroups, we found statistically significant differences in the distribution of genotypes (χ
2=6.46, df=2, p=0.04) resulting from an overrepresentation of deletion allele homozygotes among patients with late first admissions. Moreover, the allele distributions differed significantly between the two patient subgroups (p=0.03, Fisher’s exact test). Exclusion of those patients with an age at first admission below 18 years did not affect the strength of these associations. Finally, we examined the distribution of age on genotypes in the group of healthy subjects and did not find that the deletion allele was associated with a higher age.
Discussion
The present study failed to detect a significant association between the CCR5 32-bp deletion allele and schizophrenia. However, we provided evidence that this allele affects the onset of the disease. This was based upon two sets of analyses. First, we treated age at first admission as a continuous variable and found that its distribution was skewed toward higher values in patients heterozygous or homozygous for the deletion allele in comparison with wild type homozygotes. Second, we focused upon late-onset schizophrenia, defined as cases with age at first admission 40 years and older, and found an overrepresentation of genotypes homozygous for the deletion allele in this disease subgroup. Collectively, these findings suggest that the deletion allele delays the onset of schizophrenia and that two copies of this allele may postpone the onset to age 40 or beyond.
Several mechanisms could underlie the relationship between the CCR5 deletion allele and age at onset of schizophrenia. It is possible that the deletion allele is a modulating factor that protects against the generally more severe forms of early-onset schizophrenia in individuals predisposed for psychotic illnesses. Such protection could stem from an altered cytokine response to common viruses back in early childhood or prenatal life. Since the distribution of CCR5 genotypes and alleles were almost identical in patients with earlier onset forms of schizophrenia and healthy subjects, another possibility is that the deletion allele acts as a genuine susceptibility factor for late-onset forms of schizophrenia. If so, this may reflect a decreased ability of the deletion allele for clearance of common infections, leading to neuronal damages and predisposition for a specific form of schizophrenia with a late onset. Interactions between CCR5 and viruses possessing a potential for establishing persistent infections with long latency periods such as retroviruses could be of particular interest in this respect
(5).
Our finding of an association between the CCR5 deletion allele and late onset of schizophrenia should be interpreted with caution. One of the reasons for this is that the exact age at disease onset is difficult to determine, since the appearance of manifest psychotic symptoms usually is preceded by a long prodromal phase. We used age at first admission as a measure of the onset of schizophrenia. This appears to be a reasonably exact approximation to disease onset
(21). Another concern stems from the relatively low numbers of individuals homozygous for the deletion allele that might have inflated the type I error rate in the analyses of the genotype distributions.
Since the frequency of the CCR5 32-bp deletion allele differs significantly between different ethnic groups
(27,
28), population-based studies may yield spurious associations with this allele unless ethnic background is taken into consideration. We believe that the risk of erroneous results from population stratification was small in the present study, since patients as well as comparison subjects all were Caucasians from Denmark.
In conclusion, we failed to detect an association between the CCR5 deletion allele and susceptibility to schizophrenia per se, but we found evidence that this allele affects age at onset of the illness. The finding of a possible effect of the CCR5 deletion allele on the onset of schizophrenia needs to be verified by other studies.