An extensive literature on cognition in schizophrenia encompasses the nature of the impairments, the relevance of these deficits for daily functioning, and attempts to improve cognition through interventions. The vast majority of this literature has been devoted to basic cognition (e.g., impairments in attention, memory, speed of processing, etc.)
(1,
2) . In contrast, the available data on social cognition in schizophrenia is much more limited. Social cognition refers to the ability to construct mental representations about others, oneself, and relations between others and oneself
(3,
4) . Although the size of the literature is comparatively small, social cognition in schizophrenia
(5,
6) is a fast-growing area of investigation for several reasons. First, social cognition has been used to understand the formation of certain symptoms in schizophrenia, such as paranoia
(7,
8) . Second, affective neuroscience studies in schizophrenia suggest that the neural substrates of social cognition in schizophrenia are, at least partially, different from those that underlie basic cognition
(9,
10) . Third, social cognition appears to serve a key role in functional outcome in schizophrenia
(11) . Recent findings strongly suggest that social cognition mediates relationships between basic cognition and functional outcome in schizophrenia
(12 –
15) . Perhaps the best indication of the increasing interest in social cognition is that the investigators of the National Institute of Mental Health Initiative Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) group identified social cognition as one of seven domains that should be routinely assessed in clinical trial studies of schizophrenia
(16) .
A key question is whether social cognition is separable from basic cognition, and several lines of evidence suggest that it is. One line of evidence, mentioned above, is that social cognition appears to act as a mediator between basic cognition and community functioning in models of outcome
(12 –
15) . The addition of social cognition to a model improves the overall fit and demonstrates that it is not redundant with basic cognition. Additional support for the separateness of the two domains comes from a recent study that showed that schizophrenia and schizoaffective patients differed on a measure of social cognition but were indistinguishable on a variety of basic cognition measures
(17) . A direct way to examine whether these two domains are separable is to test competing models with structural equation modeling. Using baseline data from the current study on all available subjects (regardless of whether they had any subsequent assessments), we found a significantly better fit with two latent variables (one for social cognition and one for basic cognition) compared with a single latent variable that combined both domains
(18) . The two domains were clearly related to each other, but they were also separable.
Do antipsychotic medications improve social cognition in schizophrenia? Only four studies have examined this question, to our knowledge. Kee et al.
(19) reported a benefit in emotion perception for risperidone compared to haloperidol in a small (N=20) double-blind pilot study with random assignment to medication. In an open-label study without random assignment (N=52), Littrell et al.
(20) found a benefit for olanzapine compared with a variety of first-generation medications on a social perception measure. Herbener et al.
(21) found no benefit for risperidone on emotion perception in a small (N=13) crossover study of first-episode patients. In the largest study to date, Harvey et al.
(22) found that patients randomly assigned to risperidone (N=142) or quetiapine (N=124) did not improve significantly on a lone measure of emotion perception over the 8-week study period, with effect sizes of 0.11 and 0.14. The second-generation medications did not differ in their impact on emotion perception. Hence, the studies to date have involved either small samples, single measures of social cognition, or nonrandomized designs, not allowing for definitive conclusions about the influence of antipsychotic medications on social cognition or the relative benefit of first- versus second-generation medications.
Do antipsychotic medications improve basic cognition in schizophrenia? In contrast to the very limited literature on the social cognitive effects of antipsychotic medications, there is a substantial literature on the cognitive effects of second- versus first-generation antipsychotic medications. Studies have typically found that second-generation medications, when compared to first-generation medications, yield superior cognitive benefits indicated by group differences ranging from 0.2 to 0.4 SDs
(23,
24) . Of interest, several recent studies show smaller differences between first- and second-generation medications than earlier studies
(25 –
27) . The more recent studies with smaller effect sizes sometimes use lower doses of the first-generation medication. Hence, the size of the difference between first- and second-generation medications may depend partly on the dose of the first-generation medication. The results from the neurocognitive component of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study are consistent with this view because there were no differences in the neurocognitive effects of the first-generation medication (perphenazine) compared with the second-generation medications
(28) . The pattern of results in this literature raises the question as to whether the second-generation medications are truly procognitive or whether the first-generation medications are more cognitively impairing at higher doses
(29) .
The primary goal of the current study was to conduct a controlled examination of the short-term effects of two second-generation medications (risperidone and olanzapine) and a first-generation medication (haloperidol) on social cognition. A secondary goal was to examine the neurocognitive effects of these medications.
Method
Design
In this 8-week double-blind study, patients were randomly assigned to 4 mg of risperidone, 15 mg of olanzapine, or 8 mg of haloperidol. The doses were based on the prescribing practices in the Veterans Affairs Healthcare System at the time the study was designed. Two different random assignments were used. One was a simple three-way (1:1:1) random assignment to each of the three medications. However, for patients with a history of adverse experiences with haloperidol, a two-way (1:1) random assignment was used in which they were assigned to either risperidone or olanzapine. Because of these two random assignment methods, fewer subjects were assigned to haloperidol (N=20) than the other two medications (N=40 assigned to each). A total of 73 patients completed the baseline and one other assessment. Of these, 46 entered the study through the three-way random assignment, and 27 entered the study through the two-way random assignment.
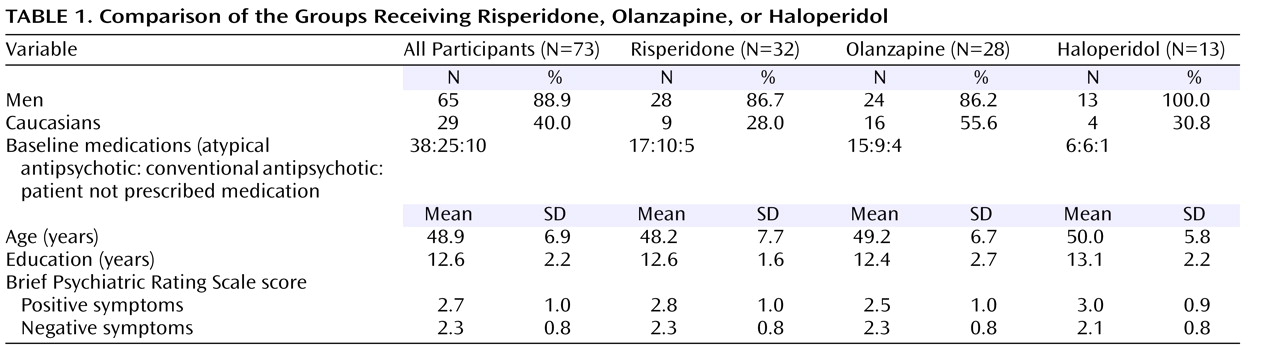
Patients were initially enrolled and tested at baseline on their prestudy medication; there was no medication washout period. A summary of the prestudy medications of the three study medication groups is presented in
Table 1 . Patients who were receiving a depot antipsychotic medication were converted to an oral administration of the same medication and were given baseline assessments 8 weeks after their last depot injection. Random assignment of patients was stratified within each site and blocked in sets of 15 (for the 3-way random assignment) to keep the groups relatively balanced throughout the study. A cross-titration procedure was used: during the first 2 weeks of the study, the patients’ baseline antipsychotic medications were reduced and discontinued as their study medication was brought to full dose by day 14. The patients were allowed the following concomitant medications: valproate, selective serotonin reuptake inhibitor antidepressants, antiparkinsonian medications (including anticholinergics and amantadine), and lorazepam. Benzodiazepines were not allowed on the day of testing. Assessments of social cognition and basic cognition were administered before the start of the study medication, 4 weeks after baseline, and 8 weeks after baseline. The study also had a psychophysiological component. The results from that component will be presented separately.
Participants
Outpatients with schizophrenia or schizoaffective disorder were recruited from treatment clinics in three Veterans Administration (VA) Healthcare Systems (see below) as well as local board-and-care facilities. The patients had to express interest in participating in a medication study but not necessarily dissatisfaction with their current medications in order to enter the study. Selection criteria included age between 18 and 60 years old, competence to provide informed consent, no identifiable neurological conditions or mental retardation, and no alcohol or substance dependence in the last 6 months. The consent and recruitment procedures were approved by the institutional review boards of the Veterans Affairs Greater Los Angeles Healthcare System, the VA Long Beach Healthcare System, and the VA San Diego Healthcare System. The participants provided their written informed consent after they read a complete description of the study.
Of the 100 participants who completed the baseline assessments, 73 completed the assessments at week 4, and 59 completed the assessments at week 8. Of the 40 risperidone-assigned patients who completed the baseline assessments, 32 completed the assessments at week 4, and 25 completed the assessments at week 8. Of the 40 olanzapine-assigned patients who completed the baseline assessments, 28 completed the assessments at week 4, and 22 completed the assessments at week 8. Of the 20 haloperidol-assigned patients who completed the baseline assessments, 13 completed the assessments at week 4, and 12 completed the assessments at week 8. Of the 73 patients who completed the baseline assessment and at least one other assessment, 32 were tested at the West Los Angeles VA, 34 were tested at the Long Beach VA, and seven were tested at the San Diego VA.
All participants met criteria for schizophrenia or schizoaffective disorder based on an interview with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID, reference
36 ). The SCID interviewers were trained to administer the SCID by an expert diagnostician from the treatment unit of the VA Mental Illness Research Education Clinical Center (MIRECC) and demonstrated agreement between their ratings and the consensus ratings of the MIRECC’s expert diagnosticians (minimum kappa coefficient of 0.80). Psychiatric symptoms were rated with the Brief Psychiatric Rating Scale (BPRS, reference
37 ). Two cluster scores were used: positive symptom cluster=conceptual disorganization, hallucination, and unusual thought content; negative symptom cluster=retardation, blunted affect, and emotional withdrawal. All BPRS raters were trained to minimum intraclass correlation coefficients of 0.80 for the BPRS based on their agreement with consensus ratings of the MIRECC’s expert diagnosticians. BPRS ratings range from 1 (not present) to 7 (extremely severe), with 4 representing the clinical threshold.
Table 1 displays demographic and symptom information about all of the participants in the analyses and separately by treatment group. The patients in the three medication groups did not differ in their demographic characteristics or symptom ratings. The symptom ratings indicate that the participants had relatively low symptom levels on average.
Measures
Social cognition
Four measures of social cognition were used: two measures of emotion perception and two measures of social perception. The two measures of emotion perception were the Facial Emotion Identification Test
(38) and the Voice Emotion Identification Test
(38) . The Facial Emotion Identification Test consists of 19 black-and-white still photographs that are presented on a videotape. The photographs, developed by Izard
(39) and Ekman
(40), were presented for approximately 15 seconds each, with an interval of 10 seconds between photographs. After each photograph, the subjects were asked to circle on an answer sheet the one emotion of six basic emotions (i.e., happy, angry, afraid, sad, surprised, and ashamed) that best described the emotion expressed by the face in the photograph. The number of items correct was the dependent measure. The Voice Emotion Identification Test consists of 21 audio recordings of statements with putatively neutral content (e.g., “Fish can jump out of the water,” and “He tossed the bread to the pigeons”). The voice tone for each statement is characteristic of one of the same six emotions included in the Facial Emotion Identification Test. The subjects listened to each sentence and then circled on an answer sheet the one emotion of the six basic emotions that best described the emotion expressed by the speaker’s tone of voice. The number of items correct was the dependent measure.
The two measures of social perception were the Half-Profile of Nonverbal Sensitivity
(41) and the Interpersonal Perception Task—15
(42) . The Half-Profile of Nonverbal Sensitivity consists of the first 110 scenes of the Profile of Nonverbal Sensitivity
(43) . Scenes of this videotape-based measure last 2 seconds and contain the facial expressions, voice intonations, and/or bodily gestures of a Caucasian woman, with one to three social cues per scene. After watching each scene, the participants were asked to select from two labels (e.g., saying a prayer, talking to a lost child); the label that best described a situation that would give rise to the social cue(s) observed. As in prior studies that have used the Profile of Nonverbal Sensitivity to assess schizophrenia patients (e.g.,
44,
45), the administration procedure was modified to reduce the measure’s demands on sustained attention and reading comprehension. Before each scene, the videotape was paused as the experimenter read the two possible labels aloud and the participant read the labels silently from a 4-by-6 index card. To ensure that the participants understood the task, a practice sample of five scenes was randomly selected from the second 110 items of the Profile of Nonverbal Sensitivity and administered before the scored scenes. The total number of items correct on the Half-Profile of Nonverbal Sensitivity was the dependent measure. The Interpersonal Perception Task—15 is a videotape-based measure that presents scenes of unscripted interpersonal interactions that range from 30 to 90 seconds in length. Scenes contain multiple social cues (e.g., facial expressions, verbalizations, voice tone/paraverbals, gestures, situational context, proxemics, haptics) displayed by one to four persons. One multiple-choice question is asked about each scene. Questions relate to varied aspects of social perception, such as intimacy (e.g., How long have they been dating?), status (e.g., Who is the boss?), and veracity (In which scene is the person telling the truth?). To reduce the measure’s demands on sustained attention and reading comprehension, the videotape was paused before each scene while the multiple-choice question and potential responses were read aloud by the experimenter and read silently by the participant. The total number of items correct on the Interpersonal Perception Task—15 was the dependent measure.
Basic cognition
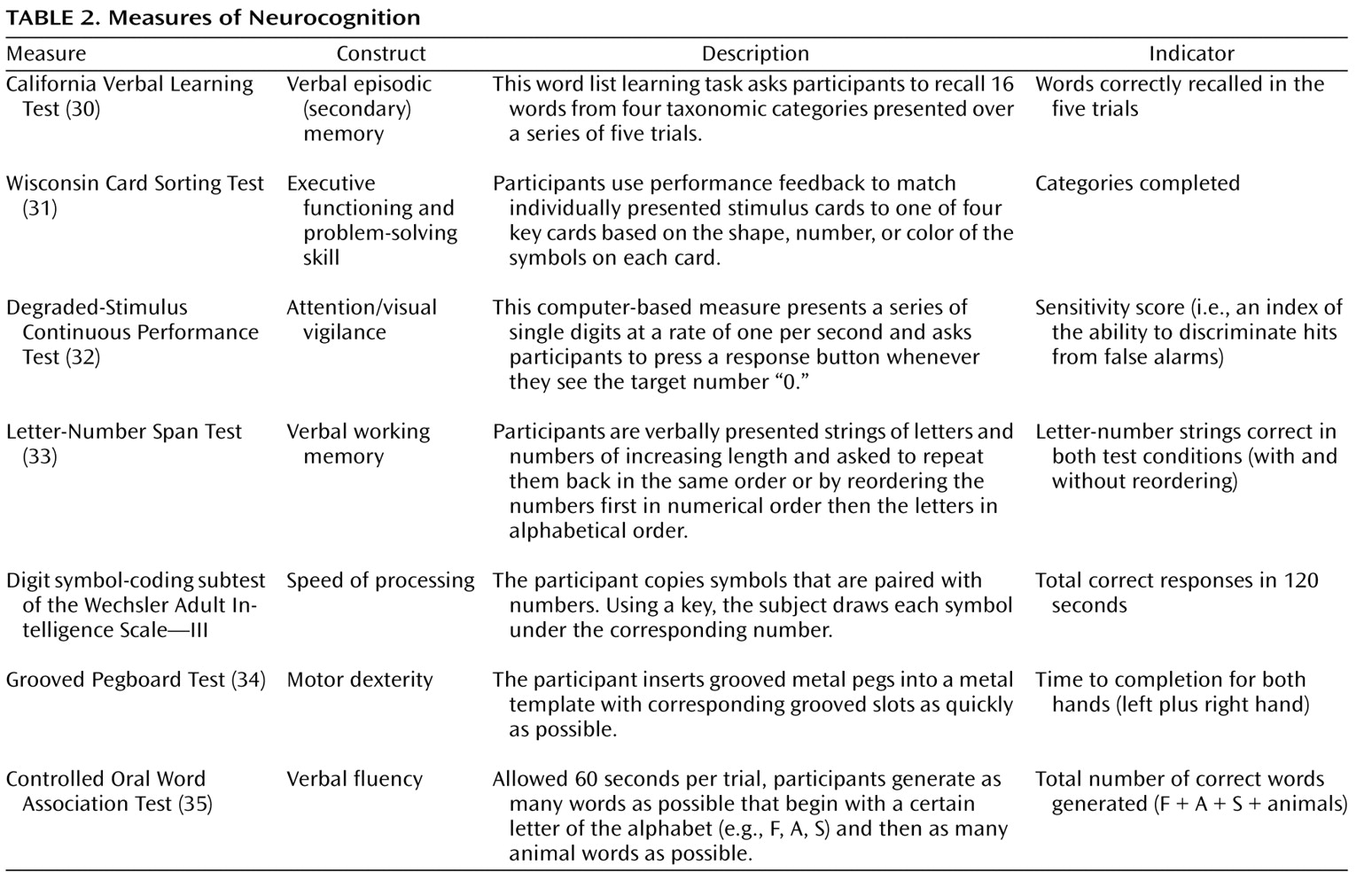
The cognitive measures included tests of attention/vigilance, executive functioning/problem solving, speed of processing, motor dexterity, verbal fluency, verbal episodic (secondary) memory, and verbal working memory. Because these measures are more familiar to most readers than the social cognitive measures, we provide a brief description of each in
Table 2 .
Statistical Analysis
A social cognition factor was defined based on a priori considerations of the construct validity of the constituent measures: Half Profile of Nonverbal Sensitivity, Interpersonal Perception Task—15, Facial Emotion Identification Test, and Voice Emotion Identification Test. To minimize the effect of missing data, the score used for the analysis was derived by standardizing the component variables based on the baseline means and standard deviations and averaging the standardized scores.
Cognition in schizophrenia is often viewed in terms of separable dimensions of cognitive deficits (e.g., reference
46 ). Adequate representations of these dimensions or factors typically require multiple measures (more than those used in the current study) of each domain. A factor analysis of baseline performance on the seven cognitive measures yielded two factors that were used for data reduction: a broad factor representing general cognitive ability and a more specific factor representing processing speed. The general cognitive ability factor was composed of scores from the California Verbal Learning Test, the Controlled Oral Word Association Test, the Degraded-Stimulus Continuous Performance Test, digit symbol-coding, Letter-Number Span, and the Wisconsin Card Sorting Test. The processing speed factor was composed of scores from the digit symbol-coding and Grooved Pegboard Test.
The primary treatment analyses used mixed-effect analyses of covariance (ANCOVAs) (SAS PROC MIXED, reference
47 ) with medication (risperidone, olanzapine, or haloperidol) as a between-groups factor and time (week 4, week 8) as a within-group factor. Baseline performance was a fixed covariate and symptom ratings (BPRS positive symptoms, BPRS negative symptoms) were time-varying covariates (i.e., the symptom ratings at 4 weeks were used as the covariates for that time point, and the ratings for 8 weeks were used as the covariates for that time point). Main effects for site were evaluated, but interactions involving site and treatment were not predicted, and we did not have sufficient power to examine them. Analyses were based on change scores from baseline derived by subtracting the baseline score from the scores at weeks 4 and 8. When baseline is included as a covariate, these are equivalent to analyses of raw scores, but this method permits testing of the significance of within-group change as well as between-groups differences, both averaged across time and at each time point. The mixed-effect analyses assume data are missing at random. The effect size estimates for each medication’s effect on social cognition involved an averaging of the week 4 and week 8 time points. These effect size estimates were calculated by using a baseline SD of 0.69, which was the mean of the standardized components of all three clusters.
Results
Medication Effects
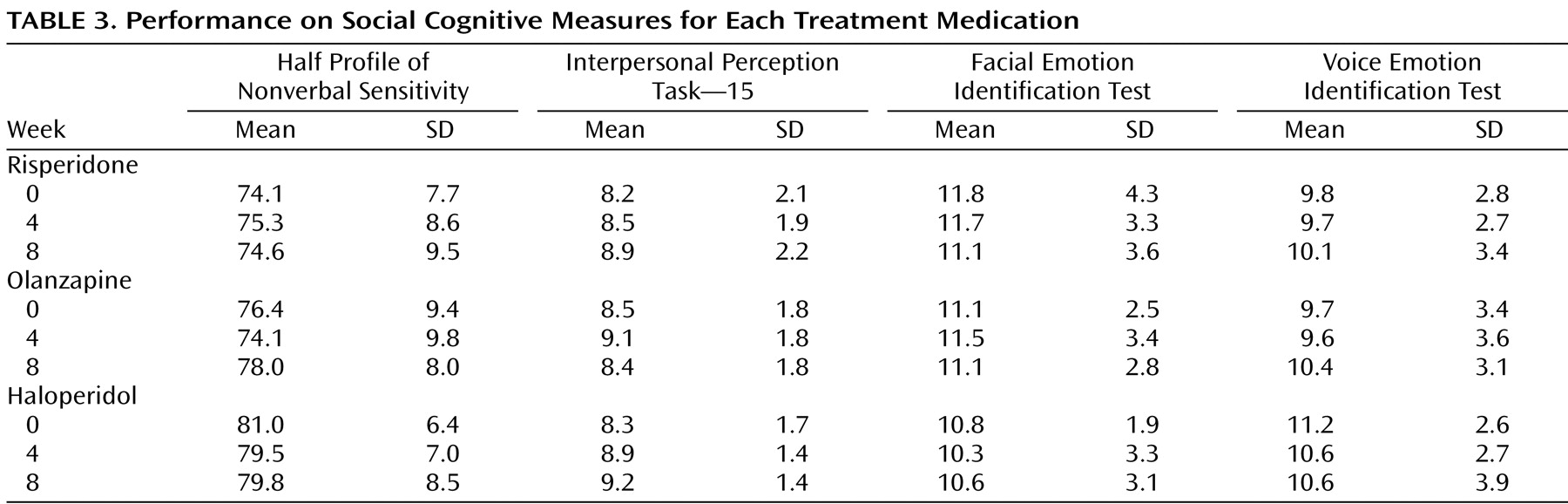
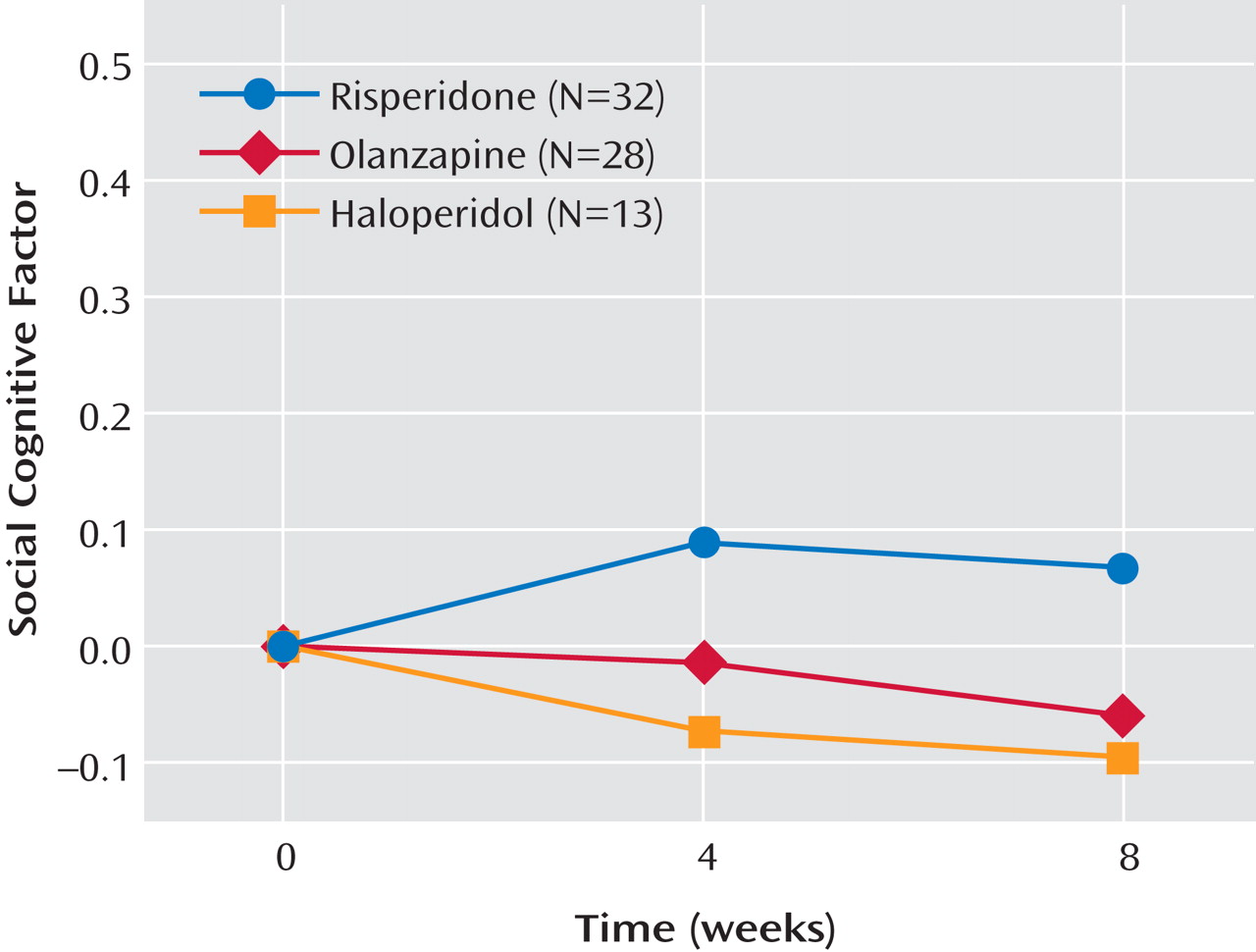
Table 3 displays the mean performance of the participants on each of the social cognitive measures separated by medication. An ANCOVA examining changes in the social cognitive cluster failed to find effects for medication, time, or the interaction of medication and time (
Figure 1 ). Effect sizes for the changes in the social cognitive cluster were small for each medication (risperidone, d=0.11; olanzapine, d=–0.05; haloperidol, d=–0.12). Because social cognition is known to overlap with basic cognition, a second ANCOVA was conducted to examine the medication effects on the social cognitive cluster controlling for basic cognition. When the two cognitive clusters were entered into the model and partialled out, differences in social cognition among the medication groups were still absent (F=0.68, df=2, 103, p=0.51).
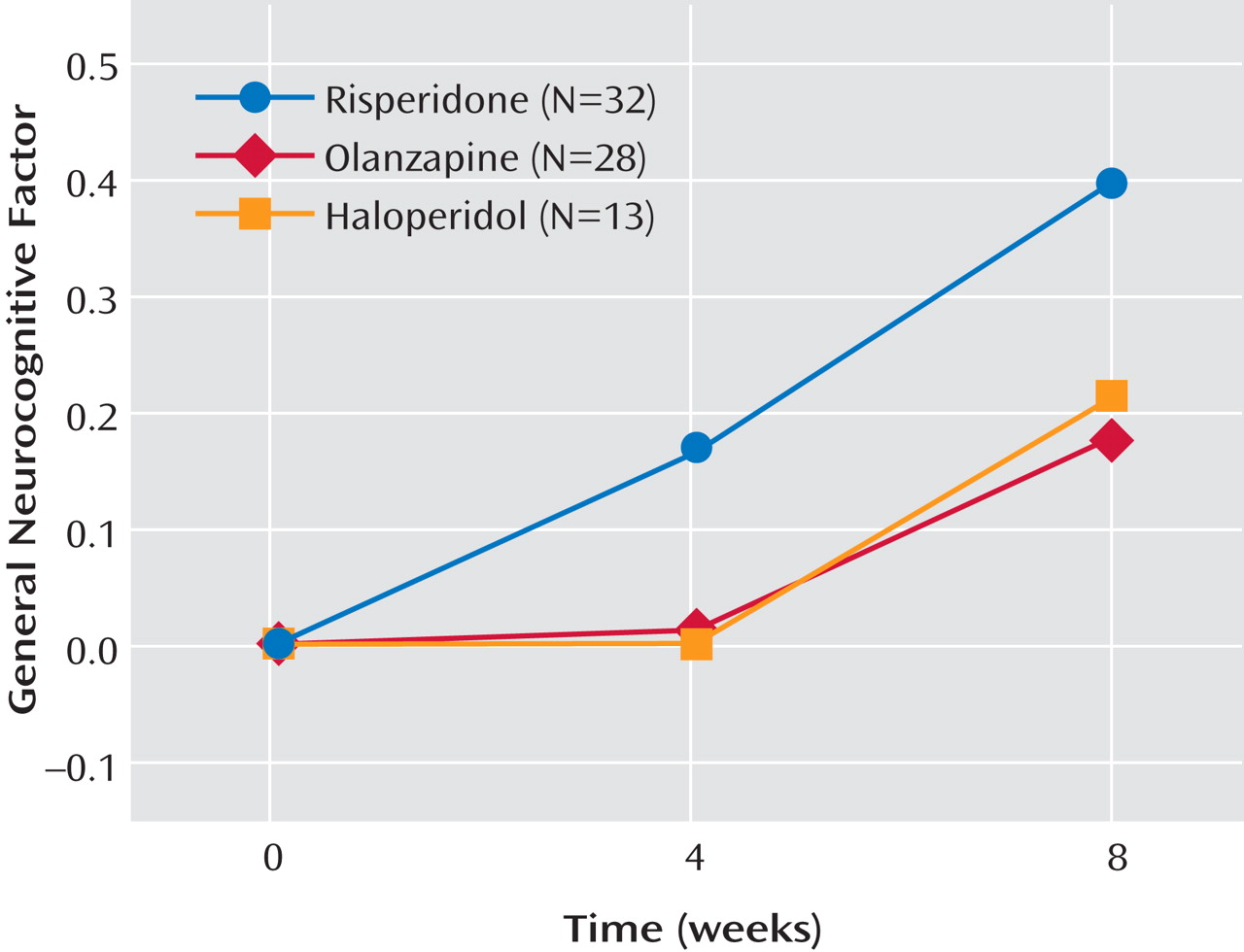
Table 4 displays the mean performance of the participants on each of the cognitive measures separated by medication. The ANCOVA for the general cognitive cluster score did not yield a main effect for medication or an interaction of medication and time. It did, however, reveal a strong main effect for time (F=20.10, df=1, 109, p<0.0001). As shown in
Figure 2, risperidone treatment appears to increase performance more over time than the other two treatments, but this effect was not significant. Across the three medication groups, change from baseline in the general cognitive factor was not significant at week 4 (adjusted mean=0.06, SE=0.06; t=0.96, df=109, p=0.34) but was significant at week 8 (adjusted mean=0.26, SE=0.06; t=4.77, df=109, p<0.0001). The ANCOVA for processing speed factor failed to find main effects for medication, time, or the interaction of medication and time (
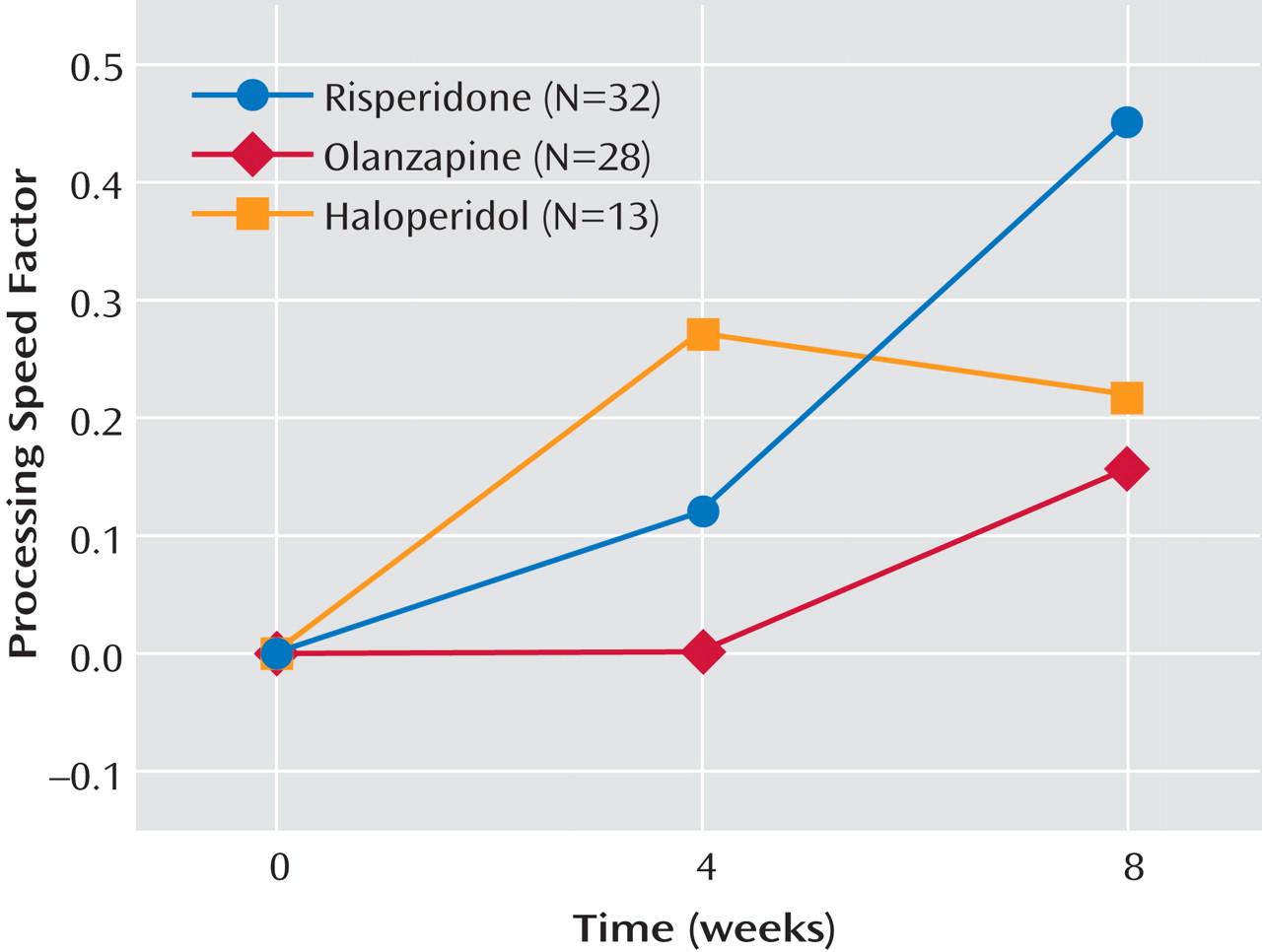
Figure 3 ). The main effect of time was a nonsignificant tendency (F=2.98, df=1, 109, p<0.10).
Attrition
Dropout rates from the study were comparable across the medication groups and not unexpected for a study of outpatients with schizophrenia. At 4 weeks, dropout rates were 20% for risperidone, 30% for olanzapine, and 35% for haloperidol (χ 2 =1.83, df=2, p=0.40). At 8 weeks, the dropout rates were 38% for risperidone, 45% for olanzapine, and 40% for haloperidol (χ 2 =0.48, df=2, p=0.79). Correlations of dropout with baseline cluster scores were very small and nonsignificant (Pearson’s r from –0.10 to –0.01; p values from 0.33 to 0.94, dfs from 96 to 97). Thus, there was no evidence of differential dropout in the treatment groups or associations with the dependent variables that might have compromised the integrity of the analyses.
Discussion
The primary goal of the study was to examine the short-term effects of two second-generation medications (risperidone and olanzapine) and a first-generation medication (haloperidol) on social cognition. The assessment battery involved a relatively broad battery of four social cognitive measures (two of social perception and two of emotion perception). Analyses revealed small effect sizes and no significant changes in social cognition associated with treatment over the 8-week study period, and there were no significant between-groups differences. When the potential influence of changes in basic cognition was statistically controlled, the findings for social cognition were essentially unchanged.
Two previous small-scale studies reported that second-generation medications improve social cognition better than first-generation medications
(19,
20) . The study by Kee et al.
(19) was distinctly different from this study because it was conducted with treatment-resistant inpatients and the dosing of the first-generation medication was relatively high (6 mg of risperidone and 15 mg of haloperidol). The high dosing of the comparator medication may have influenced the results in that study
(27,
29) . The results from Littrell et al.
(20) may have been influenced by the fact that the study was open label and nonrandomly assigned. The current null results are consistent with the finding of Herbener et al.
(21) that a second-generation medication (risperidone) did not improve emotion recognition in a small sample of first-episode patients. Likewise, the currently observed small effect sizes for changes in social cognition influenced by second-generation medications (risperidone and olanzapine) are consistent with the small effect sizes for emotion perception influenced by second-generation medications (risperidone and quetiapine) observed by Harvey et al.
(22) .
A secondary goal of this study was to examine the effects of the first- and second-generation medications on basic cognition. This is an important topic, but we considered it secondary because the question has been considered in larger studies, most recently in the cognitive component of CATIE. No medication-specific changes in general cognitive ability or processing speed were observed. However, general cognitive ability improved over time for each medication, an effect that may be attributable to the increased test familiarity and practice effects from repeated testing in an 8-week period. As mentioned above, most studies have found that second-generation medications provide greater cognitive benefits than first-generation medications
(24), although the CATIE study did not
(28) . The current study’s failure to find a second- versus first-generation medication effect for cognition may be because of the relatively small number of patients assigned to haloperidol (a result of the two different random assignment approaches). Also, the dose of haloperidol used in this study (8 mg), although not very low, is lower than that used in many of the previous studies that reported second-generation versus first-generation differences. Using lower doses of the comparator first-generation medication may minimize the differences between first- and second-generation medications
(26,
27) . Moreover, the assignment of patients with a history of adverse responses to haloperidol to either risperidone or olanzapine may have provided haloperidol a small advantage over the second-generation medications if history of adverse response is associated with recalcitrant cognitive impairments.
The current study had some notable limitations. First, it had limited statistical power because of a modest group size and two random assignment paths. Second, the study lasted only 8 weeks. Medication-influenced changes in basic and social cognition may require more time. Third, the study did not assess other social cognitive areas such as theory of mind and emotion processing. Despite these limitations, we believe this study is a very thorough investigation of the effects of antipsychotic medications on social cognition. The results show small effect sizes and no significant differences among the medications or within each medication group over time. The findings do not provide grounds for optimism for finding differential medication effects on social cognition. In all likelihood, the important social cognitive dimension of functional outcome in schizophrenia will eventually be treated with a combination of cognition-enhancing medications and nonpharmacological training interventions.