Depressed adults exhibit altered neural and physiological responses to emotional material, particularly greater activation in limbic regions, such as the amygdala, and dysfunction in prefrontal cortical systems that modulate limbic activity
(2,
3) . However, other studies have reported reduced neural responses to emotional stimuli in adults with major depression, possibly reflecting “emotional blunting”
(4,
5) . These central disruptions are often reflected in peripheral physiological indicators of emotional information processing, such as event-related potentials or eye blinks (e.g., references
6,
7) .
Few studies have examined physiological reactivity to emotional stimuli in depressed children or adolescents, and the extent to which models derived from adult studies can be applied to children is unclear. Initial data suggest developmental differences. For example, Thomas et al.
(8) found that depressed children had a blunted amygdala response to fearful faces relative to healthy comparison children, and others
(9) have reported a blunted sensitivity to affective feedback in depressed adolescents. Thus, it is possible that whereas adults are characterized by hyperreactivity to emotional stimuli, depressed children may have decreased reactions.
Pupillometry is one promising method for investigating the temporal pattern of emotional reactions. The pupil becomes more dilated in response to stimuli requiring greater cognitive load or emotional intensity
(10,
11) . The pupil is innervated by brain structures involved in both cognitive and emotional processing. Inhibition of the pupillary constrictor muscle occurs through parasympathetic innervation of the Edinger-Westphal nucleus, which receives extensive inputs from cortical and limbic regions. Stimulation of the pupillary dilator muscle occurs through a hypothalamic pathway that also receives corticolimbic inputs. Thus, stimulation of limbic regions, such as the amygdala, increases pupil dilation
(12), as does stimulation of the midbrain reticular formation
(13), which receives afferent projections from the frontal cortex and sends efferent projections to the ocular motor nuclei, particularly structures such as the anterior cingulate cortex, which are implicated in emotion regulation
(14) . Concurrent pupillary and functional magnetic resonance imaging (fMRI) studies suggest that pupil dilation provides a summative index of task-related cognitive-affective brain activity
(15) .
Pupil dilation also provides information about the time course of brain activation in response to a stimulus. The pupil remains dilated as long as the processing demand persists, and because pupil dilation can be sampled every 16 msec, it provides a dynamic measure of changes in cognitive-affective load following exposure to the stimulus. We have shown
(11,
16) that in adults with depressive disorders, pupil dilation in tasks requiring identification of the valence of emotional words is increased and sustained for up to 30 sec after behavioral responses.
In this study, we investigated pupil dilation to emotional stimuli in depressed children and adolescents during a valence identification task. Our first goal was to replicate findings of altered pupil dilation in response to emotional words in a child and adolescent sample with depression. Our second goal was to address whether this physiological response in the laboratory directly applies to real-life affective functioning. We examined associations between pupil dilation and children’s affect ratings during social interactions collected via ecological momentary assessment, an ecologically valid method of gathering representative data on affect and behavior in natural environments.
Results
Demographic Characteristics and Reaction Times
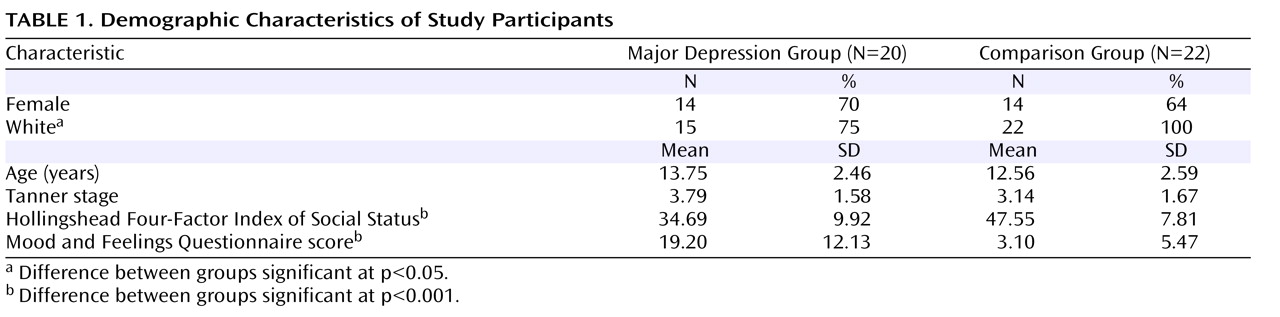
There were no group differences in age or gender. The families of the children in the major depression group were lower in socioeconomic status and more likely to have minority status than the families of children in the comparison group. There were no differences in harmonic mean reaction times by valence and no diagnostic group-by-valence interactions, although children in the major depression group responded more slowly than those in the comparison group to all words (t =2.29, df=40, p<0.05; Cohen’s d=0.64).
Pupil Dilation
Hypotheses were tested using mixed-effects models with an autoregressive covariance structure (AR1) using pupil dilation waveforms as the dependent variable. Models included mean pupil dilation at each second for each valence per subject, with subject treated as a random effect and time and valence as repeated measures. Fixed effects included diagnostic group, valence, segment, and all interactions. Segment 1, the “early” segment, was defined as the latter 3 sec of the period during which the word was on-screen (3 sec to 6 sec), and segment 2, the “late” segment, was defined as the latter 3 sec of the period during which the word was off-screen (9 sec to 12 sec). Straightforward methods for calculating statistical power for repeated-measures mixed-effects models are not yet available. Using standard general linear model methods, our sample of 42 provides power ≥0.80 to detect only large effect sizes; however, as mixed-effects models take advantage of all degrees of freedom for repeated measurements, this is likely a conservative estimate.
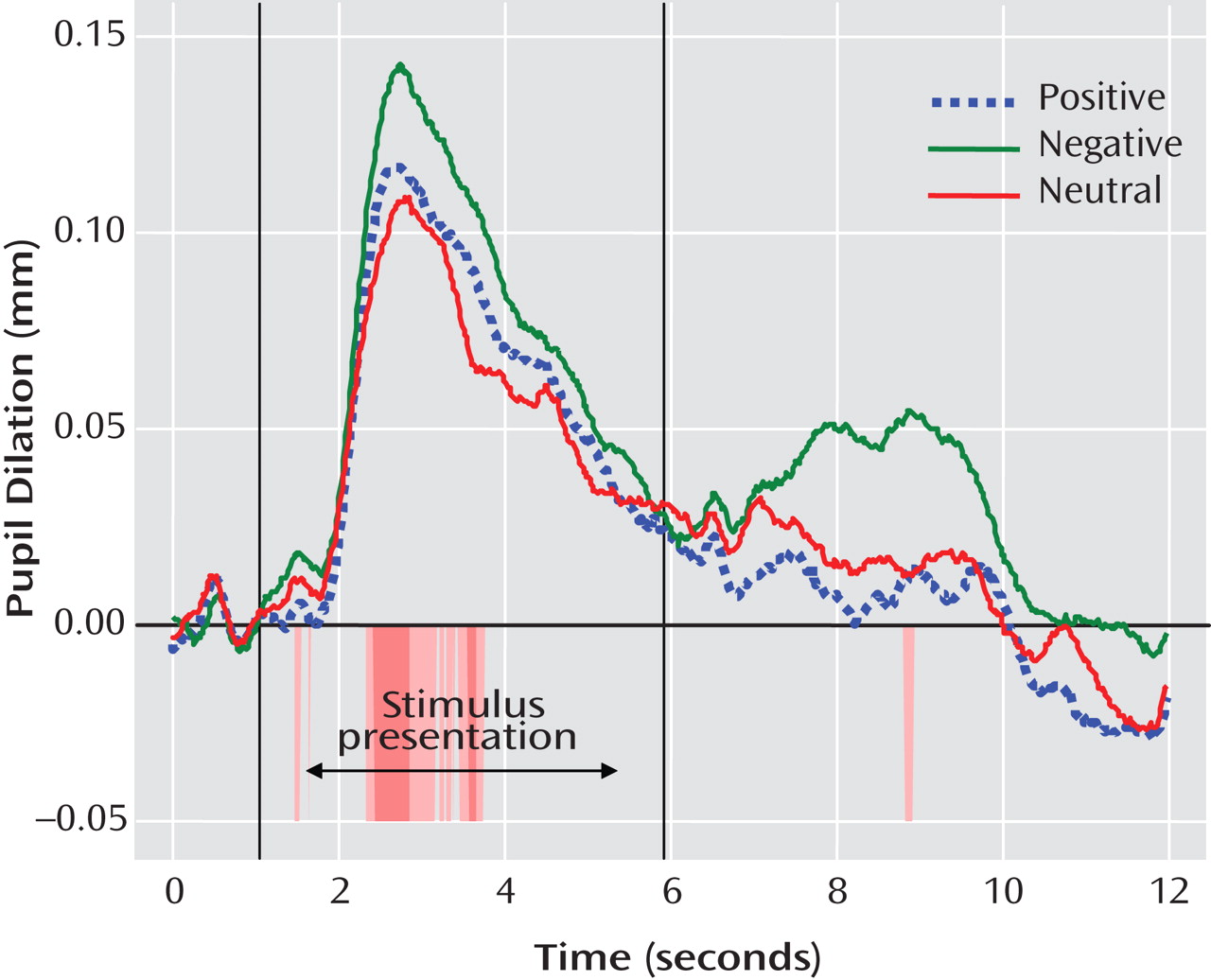
Mixed-effects analyses indicated main effects for valence (F=7.25, df=2, 674, p<0.01) and segment (F=26.74, df=1, 312, p<0.001), which were qualified by a group-by-valence interaction (F=8.66, df=2, 674, p<0.001) and a group-by-valence-by-segment interaction (F=7.73, df=2, 681, p<0.001). Because the two groups differed in mean socioeconomic status and proportion who had minority status, we ran the mixed-effects model covarying socioeconomic status and minority status. We also included a covariate for age to account for the wide age range of participants. In the model including these three covariates, effects remained significant for valence (F=16.37, df=2, 605, p<0.001), segment (F=25.43, df=1, 273, p<0.001), and the group-by-valence-by-segment interaction (F=6.71, df=2, 612, p<0.01), and the diagnostic group-by-valence interaction was no longer significant (F=1.74, df=2, 605, p=0.18). Pairwise comparisons of estimated marginal means revealed that pupil dilation was greater during segment 1 (estimated marginal mean=0.07 mm) than segment 2 (estimated marginal mean=0.01 mm; Cohen’s d=0.59) and was greater to negative words (estimated marginal mean=0.05 mm) than to positive (estimated marginal mean=0.03 mm) or neutral words (estimated marginal mean=0.03 mm; Cohen’s d=0.21; see
Figure 2 ).
The diagnostic group-by-valence-by-segment interaction was probed by examining group effects at each time point along the waveform for each valence. To control type I error, we used Guthrie and Buchwald’s technique
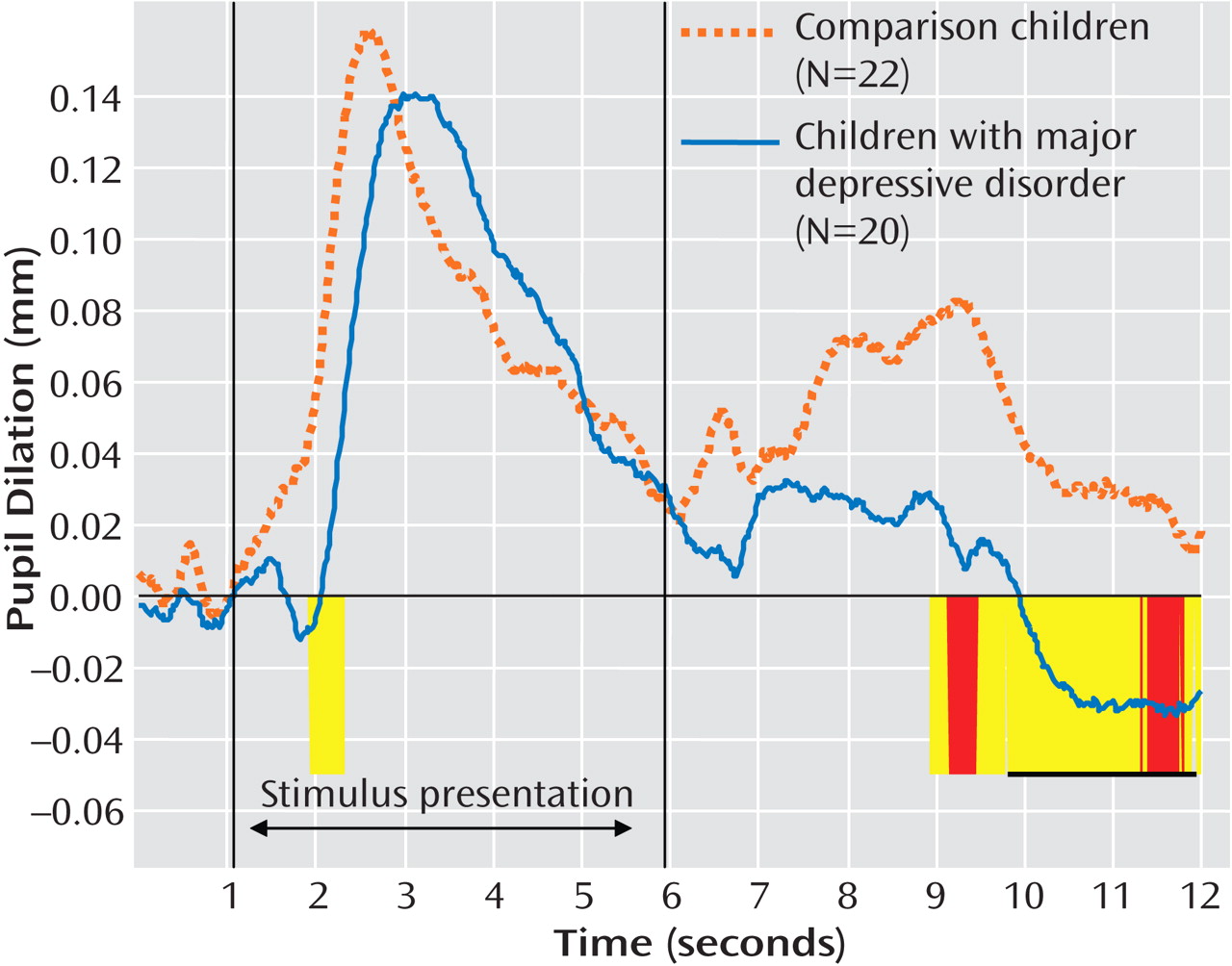
(27) to identify regions of the waveform over which an entire series of contiguous point-by-point tests, each significant at p<0.1, could be considered significant at p<0.05, given the temporal autocorrelation of the waveform. This analysis revealed that diagnostic groups differed primarily in their pupil dilation following negative words, after the word was no longer on-screen. As shown in
Figure 3, children with major depression showed less late pupil dilation relative to comparison children from approximately 9 sec to 12 sec after the beginning of the trial (8.93–9.78 sec: t=2.02, df=40, p<0.05; Cohen’s d=0.62; 9.80–11.90 sec: t=2.08, df=40, p<0.05, Cohen’s d=0.64).
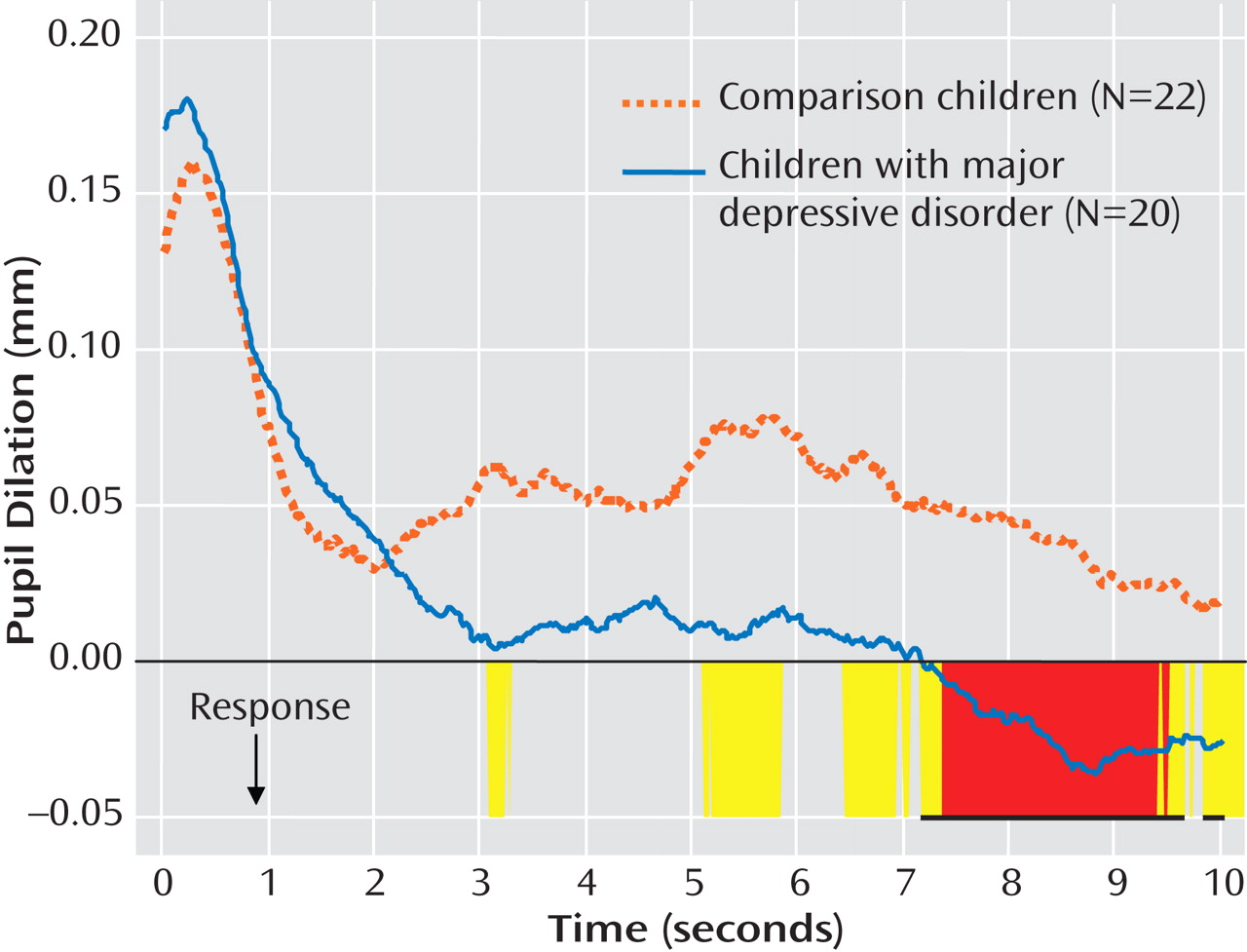
Because children in the major depression group had slower reaction times than children in the comparison group, reaction-time-aligned waveforms were examined (
Figure 4 ). Participants in the major depression group had less pupil dilation to negative words relative to those in the comparison group for the majority of the period 5–10 sec after their response (t=1.83–2.46, df=40; p=0.02–0.07; Cohen’s d=0.57–0.76).
The two groups did not differ on pupil dilation to neutral or positive words at any point along the waveform, with the exception of a lower degree of dilation to positive words in comparison children relative to depressed children during the last second of the trial (t=–2.53, df=40, p<0.05, Cohen’s d=–0.78).
Late Pupil Dilation and Depressive Symptoms With Comorbid Anxiety
As expected, children in the major depression group had more depressive symptoms than comparison children (
Table 1 ). Depressive severity was inversely related to late pupil dilation to negative words, such that children with greater depressive severity had less late pupil dilation to negative words (r= –0.35, p<0.05). Depressed children with and without comorbid anxiety disorders both displayed less late pupil dilation to negative words (t=0.76, df=18, p=0.46; Cohen’s d=0.34).
Correlation of Late Pupil Dilation With Self-Reported Emotions in the Natural Environment in Everyday Life
In the ecological momentary assessment, children in the major depression group reported higher levels of negative affect (t=3.64, df=33, p<0.01; Cohen’s d=1.27) and had a lower ratio of positive to negative affect (t=–2.62, df=33, p<0.05; Cohen’s d=0.90) in the natural environment during the weekend preceding the laboratory visit relative to comparison children. Negative affect (r=0.83, p<0.001) and the ratio of positive to negative affect (r=–0.57, p<0.001) were highly related to severity of depression, although positive affect was less strongly related to severity of depression (r=–0.32, p=0.07).
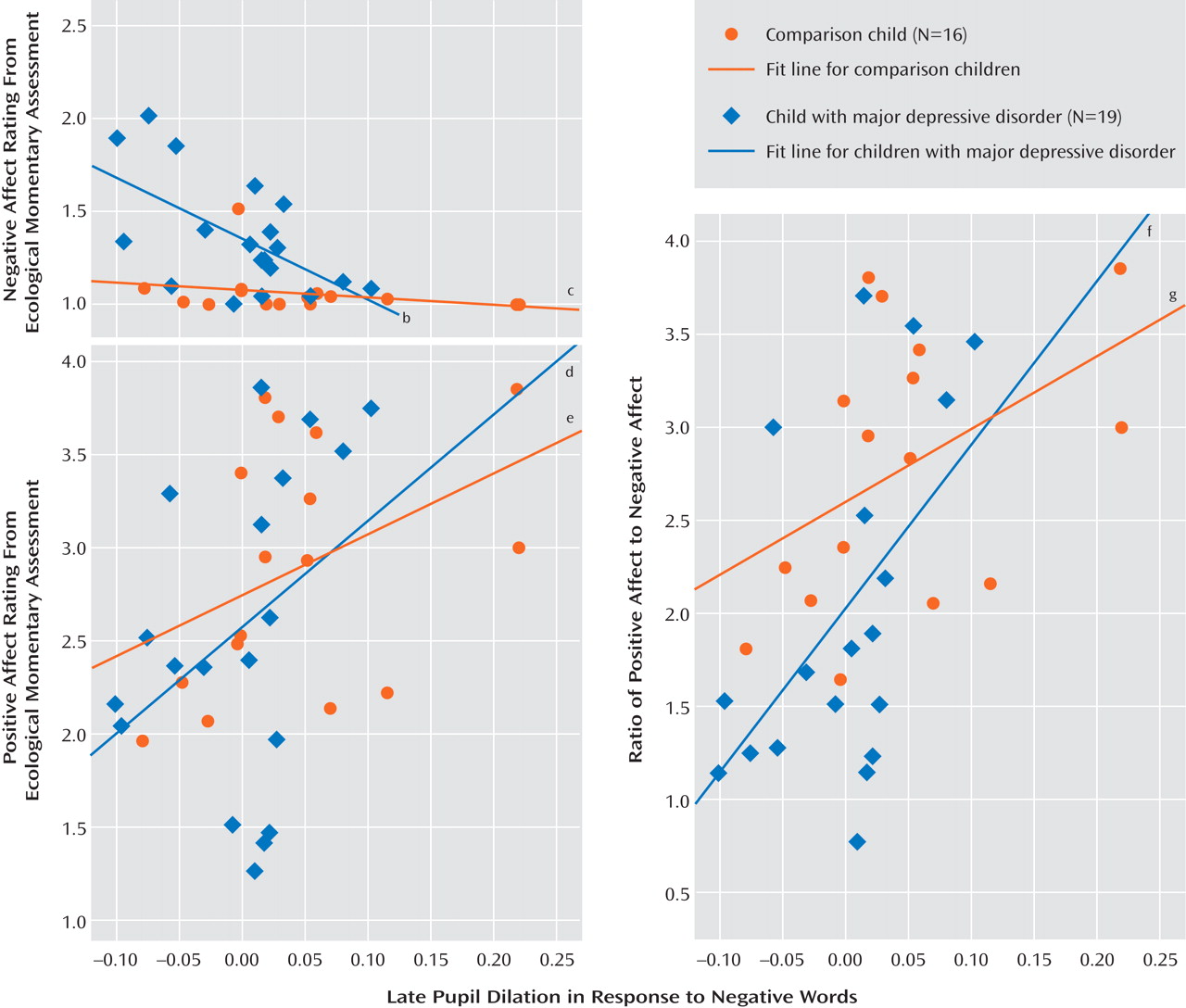
Late pupil dilation after negative words was strongly associated with positive and negative affect in the natural environment reported via ecological momentary assessment (
Figure 5 ). Participants with less late pupil dilation to negative words reported higher levels of global negative affect (r=–0.49, p<0.01) and lower levels of global positive affect (r=0.41, p<0.05) and had a lower ratio of positive to negative affect (r=0.53, p<0.01). Hierarchical linear regressions indicated that there were no interactions between pupil dilation and diagnostic group in positive affect ratings or ratio of positive to negative affect, but there was a pupil dilation-by-group interaction in predicting negative affect (ΔR
2 =0.11, F=7.16, p<0.05, Cohen’s f
2 =0.23). As shown in
Figure 5, post hoc tests revealed that late pupil dilation to negative words was associated with negative affect in children with major depression (β=–0.59, p<0.01) but not comparison children (β=–0.25, p=0.36). This difference is likely driven by low levels of variability in negative affect in ecological momentary assessments in comparison children (see
Figure 5 ).
Discussion
To our knowledge, this is the first study to assess pupil dilation in response to emotional stimuli in children and adolescents with major depression. Pupil dilation in children was greater after negative words than neutral or positive words, which suggests that pupil dilation in response to negative words during a valence identification task can index emotional information processing in pediatric populations. Group differences in pupil dilation to negative words occurred long after the stimulus onset, 5 to 10 sec after the participant’s response. Participants with major depression showed the expected initial reaction to negative words but, contrary to our expectations, showed diminished pupil dilation to negative words compared with children in the comparison group after the word was no longer on-screen. This effect was not observed with neutral or positive words. Moreover, the diminishment in late pupil dilation to negative words was more marked in children with greater severity of depressive symptoms.
This normal initial pupillary response paired with a later blunted or decreased pupillary response could indicate a more dramatic decrease in cognitive-affective resources devoted to processing negative emotional words after a negative word is initially processed in depressed children than in nondepressed children. Multiple alterations in emotional reactivity or regulation could be responsible for such decreased resource recruitment and should be investigated in future research. For example, decreased cognitive-affective load could reflect avoidance of emotional information or affective blunting or could be the consequence of earlier overcompensatory regulation. Since the pupil is innervated by both sympathetic and parasympathetic fibers, it is not possible to draw conclusions about neural mechanisms underlying this finding. Potential brain mechanisms could include blunted or diminished reactivity of emotional circuitry (e.g., the amygdala). Alternatively, prefrontal or anterior cingulate regulatory structures may fail to engage or may, after initial engagement, yield decreased sustained limbic activity. This lack of neural specificity is a limitation to the pupillometry approach that could be addressed in future research by use of concurrent collection of pupillometric and neuroimaging data, which is now available in many fMRI laboratories and can be used to better delineate the time course of activation in specific brain regions (see reference
15 ).
Although these interpretations remain speculative until the underlying neural circuitry can be better delineated, they are consistent with clinical and research reports. For example, devotion of less resources to processing negative emotional information after the initial response in depressed children is consistent with clinical reports of patients who are easily upset but “shut down” emotionally when facing too much arousal. The possibility that this pattern of findings reflects avoidance processes is consistent with evidence that adolescents with more severe depressive symptoms use more avoidance and disengagement strategies to regulate negative emotions
(28,
29) . The hypothesis that limbic reactivity may be blunted in depressed children is consistent with the finding by Thomas et al.
(8) of blunted amygdala reactivity to fearful faces in depressed children and the finding by Jazbec et al.
(9) of blunted affective sensitivity in adolescents with major depression during an antisaccade task. Our potential explanations are also consistent with reports of reduced neural and physiological responses to emotional stimuli in adults with major depression (e.g., references
4,
5,
30) .
The ecological momentary assessment data also suggest that the lower pupil dilation response is related to problems in emotional reactivity and/or regulation in everyday life. We found that children with lower levels of late pupil dilation in response to negative words in the laboratory reported higher levels of negative emotion and lower levels of positive emotion and had a lower ratio of positive to negative emotion in their everyday lives. The relationship between late pupil dilation to negative words and daily negative emotion was particularly pronounced in depressed children, suggesting that this tool may have particular utility in understanding emotional functioning in this population. These ecological momentary assessment findings show that our measure of pupil dilation to negative words has ecological validity and has implications for children’s everyday emotion regulation and social functioning.
Our finding that group differences in pupil dilation to negative words appear primarily in the late processing period is consistent with findings from studies of adults with major depression
(11,
16) . However, the direction of this effect differed from that of the adult studies, in which major depression is associated with
increased late pupil dilation to emotional words
(11,
16) . One possible interpretation of this finding is that children and adolescents with affective disorders may have an ability that is lost later in development to “shut off” or avoid the processing of negative information. By blunting, avoiding, or overregulating negative emotions, the depressed child would lose the opportunity to develop and practice more adaptive skills for tolerating and regulating negative emotions, potentially leading to greater difficulty managing emotion as an adult. Another possibility is that the increased late pupil dilation observed in adults is indicative of ruminative processes
(11) that may be less developed in children and young adolescents.
This potential developmental difference is consistent with evidence that patterns of emotional processing change across development, including several instances in which these patterns have been reported to reverse direction. For example, amygdala reactivity to affective stimuli has been shown to be increased in depressed adults but decreased in depressed children
(8), and activity to fearful versus neutral faces has been shown to be increased in adults but decreased in children
(31) . There is also evidence that brain regions involved in emotion regulation undergo functional and anatomical reorganization during puberty and continue to mature into early adulthood (see reference
32 ) and that adolescents and adults differ in the way they allocate neural resources to emotional information
(33) . Cross-sectional research with larger numbers of children and adults, as well as longitudinal research, is needed to better delineate the trajectory of pupillary responses across development.
Another possibility is that the difference between our findings and findings with adults is a function of stimulus timing rather than development. Because children take longer to read words than adults, we increased the word presentation period from 150 msec
(11,
16) to 5,000 msec. Emerging evidence suggests that patterns of emotional processing may depend on whether stimuli are presented for long enough to engage effortful processing (approximately >500 msec; see reference
34 ). For example, anxious children and adults have been shown to display vigilant reactions to quickly presented or subliminal threat stimuli but avoidant reactions to longer presentations
(34 –
36) . Similarly, children with recurrent abdominal pain have shown attentional biases toward pain-related words presented subliminally (20 msec) and attentional biases away from pain-related words presented for 1,250 msec
(37) . The relatively long word presentation in our study may have allowed children sufficient time to engage top-down regulatory resources. Research is needed to compare children’s pupillary response to words presented for both brief and long durations.
Several limitations of this study should be noted. First, the study was powered sufficiently to detect only large effect sizes, and it was underpowered to detect group-by-development interactions. Second, as described above, pupillometry does not provide direct information about specific brain regions involved in the observed response. Yet, significant conclusions may be drawn from this study given its unique strengths, which include a rigorously diagnosed clinical sample of depressed children and adolescents, strong temporal precision regarding the pattern of early and late emotional processing afforded by pupil dilation (not available with self-report), and sampling of ecologically valid information about children’s affective functioning in their daily life. If replicated, these findings could have clinical and methodological implications, as pupillometry is a practical and inexpensive method for collecting information about emotional processing that could be administered in clinical settings and is feasible with pediatric populations. Research is needed to evaluate whether this pupillary response could be a marker of risk for depression in vulnerable children or whether it is a state marker associated with the experience of depression.