Several factors are important contributors to the pathogenesis of white matter hyperintensities, particularly age
(5) and medical comorbidity. Comorbid medical conditions such as hypertension
(6), diabetes mellitus
(7), cardiovascular disease
(8), and higher Framingham Study risk factor scores
(8,
9) are especially significant. While white matter hyperintensities are more prevalent in patients with vascular risk factors, they are also noted to occur at rates of up to 60% in healthy elderly patients
(10) . Although studies examining the occurrence of white matter hyperintensities in depressed subjects have matched subjects for age, most studies have used either healthy comparison subjects or a comparison sample of convenience, rather than matching for vascular risk factors.
Results
Table 1 shows demographic variables for depressed patients and comparison subjects, including age, education, gender, race, depression symptom severity on the Montgomery-Åsberg Depression Rating Scale, and vascular risk factor score as defined by the Framingham Study
(15) . As shown in
Table 1, depressed subjects had higher Montgomery-Åsberg Depression Rating Scale scores than comparison subjects, but the groups did not differ significantly on other variables. We compared whole brain volumes of white matter, gray matter, and white matter hyperintensities between depressed and comparison subjects.
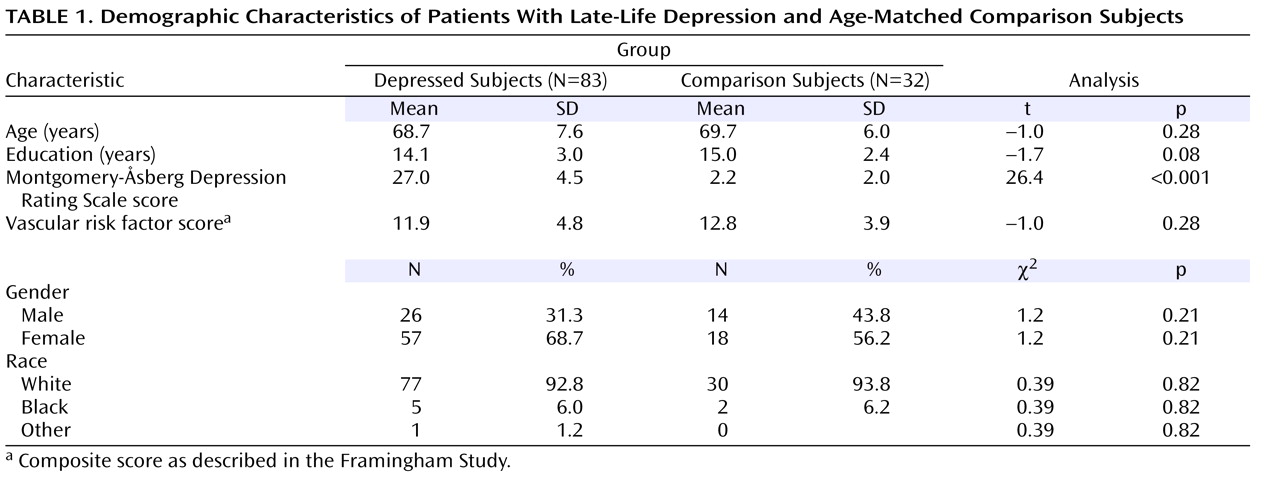
Figure 1 shows T1-weighted, T2-weighted, and segmented images for a subject with minimal white matter hyperintensities and a subject with significant white matter hyperintensities. A multivariate analysis of variance (MANOVA) was conducted to examine differences between the depressed and comparison groups in the three segmented measures of whole brain volume. The omnibus MANOVA was not significant (p>0.05), suggesting that whole brain volumes of white matter, gray matter, and white matter hyperintensities did not differ between groups for any of these three volume measurements.
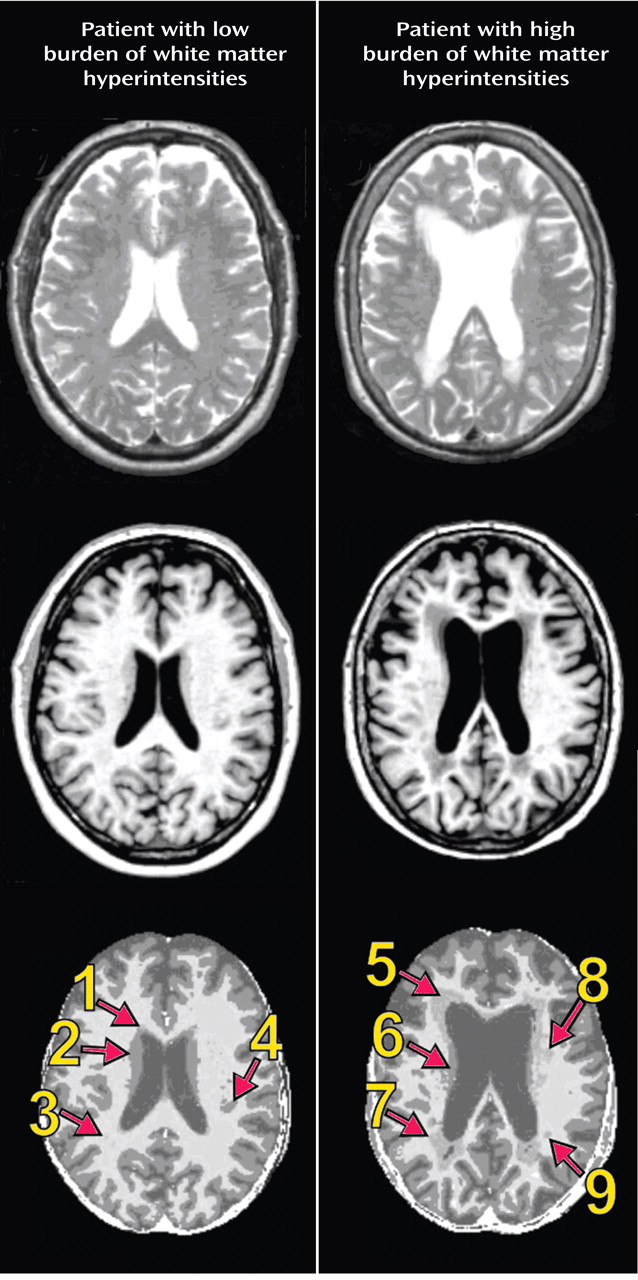
We then examined differences between the comparison and depressed groups in the prevalence of regional white matter hyperintensities. This analysis identified seven regions that met criteria and differed significantly between the two groups in white matter hyperintensity volumes (
Figure 2 ). The following regions were identified: 1) right superior longitudinal fasciculus division 1/fronto-occipital fasciculus; 2) right superior longitudinal fasciculus divisions 2 and 3; 3) a second region in the right superior longitudinal fasciculus divisions 2 and 3; 4) left superior longitudinal fasciculus divisions 2 and 3; 5) left uncinate fasciculus/frontal operculum; 6) right extreme capsule; and 7) right inferior longitudinal fasciculus. For each of these lesions, the white matter fasciculus involved and the relevant overlying cortical region are described in
Figure 2 .
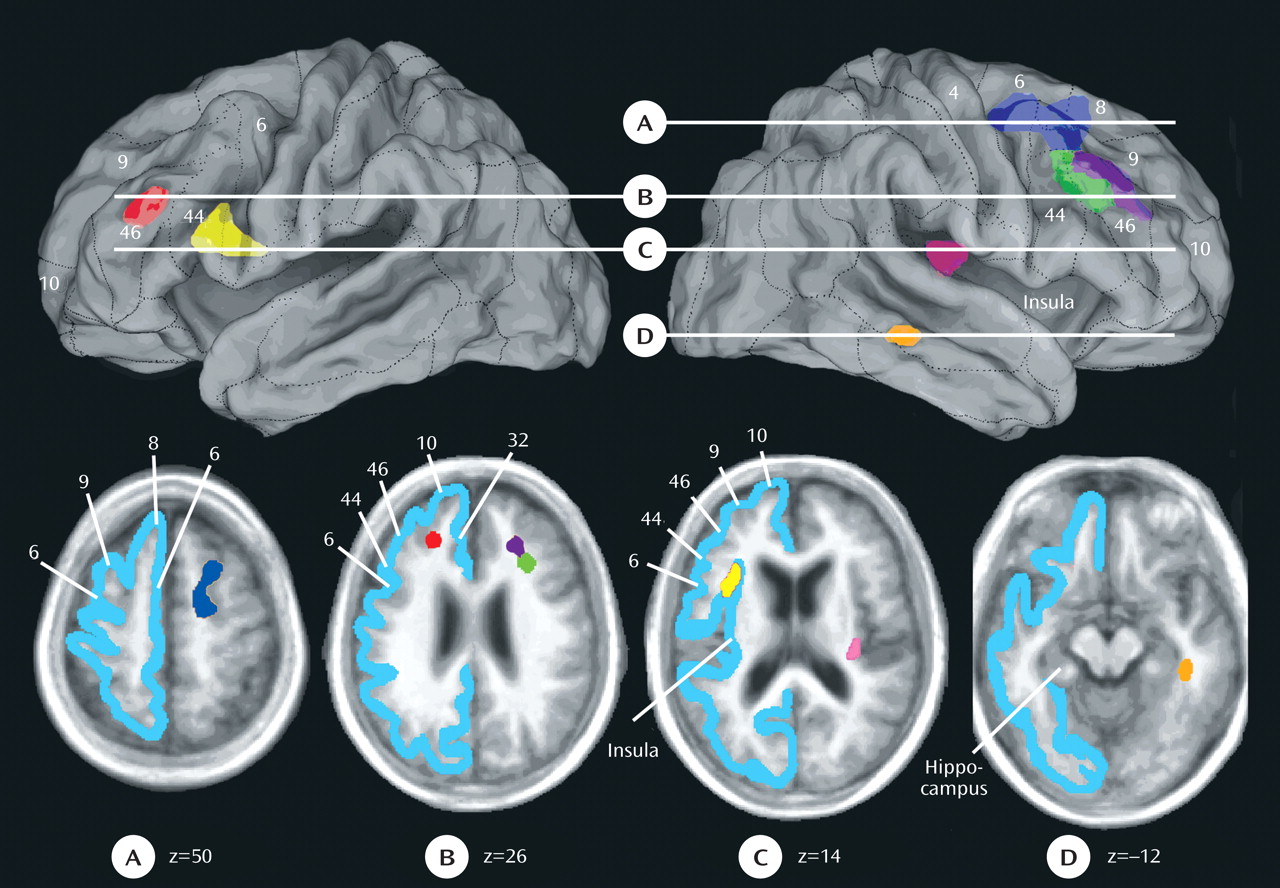
The mean white matter hyperintensity volume within each region in both depressed and comparison subjects was plotted and is shown in
Figure 3 . The effect sizes for these differences were fairly similar across the seven regions and were as follows: 1) right superior longitudinal fasciculus 1/fronto-occipital fasciculus: Cohen’s d=0.43; 2) right superior longitudinal fasciculus divisions 2 and 3: Cohen’s d=0.39 (first region); 3) right superior longitudinal fasciculus divisions 2 and 3: Cohen’s d=0.37 (second region); 4) left superior longitudinal fasciculus divisions 2 and 3: Cohen’s d=0.33; 5) left uncinate fasciculus: Cohen’s d=0.37; 6) right extreme capsule: Cohen’s d=0.40; and 7) right inferior longitudinal fasciculus: Cohen’s d=0.43.
We then examined the relationship between the volumes of white matter hyperintensity within each of the seven regions and performance in the five different a priori domains of cognitive function
(20) in the depressed and comparison subjects separately. Among the depressed patients, only three correlations were significant, and all three were with executive function as follows: 1) right superior longitudinal fasciculus 1/fronto-occipital fasciculus (r=–0.31, p=0.005); 2) left superior longitudinal fasciculus divisions 2 and 3 (r=–0.30, p=0.008); and 3) left uncinate fasciculus (r=–0.30, p=0.008). The magnitude of these correlations changed only minimally (with one going up) when age and education were partialled out. These changes were as follows: 1) right superior longitudinal fasciculus 1/fronto-occipital fasciculus (r=–0.35, p=0.002); 2) left superior longitudinal fasciculus divisions 2 and 3 (r= –0.28, p=0.02); and 3) left uncinate fasciculus (r=–0.26, p=0.02). Among comparison subjects, there were no significant correlations. Fisher’s r-to-z transformations indicated that there was a tendency toward the correlation between the right superior longitudinal fasciculus 1/fronto-occipital fasciculus and executive function being significantly more negative in patients relative to comparison subjects (z=–1.6, p=0.06).
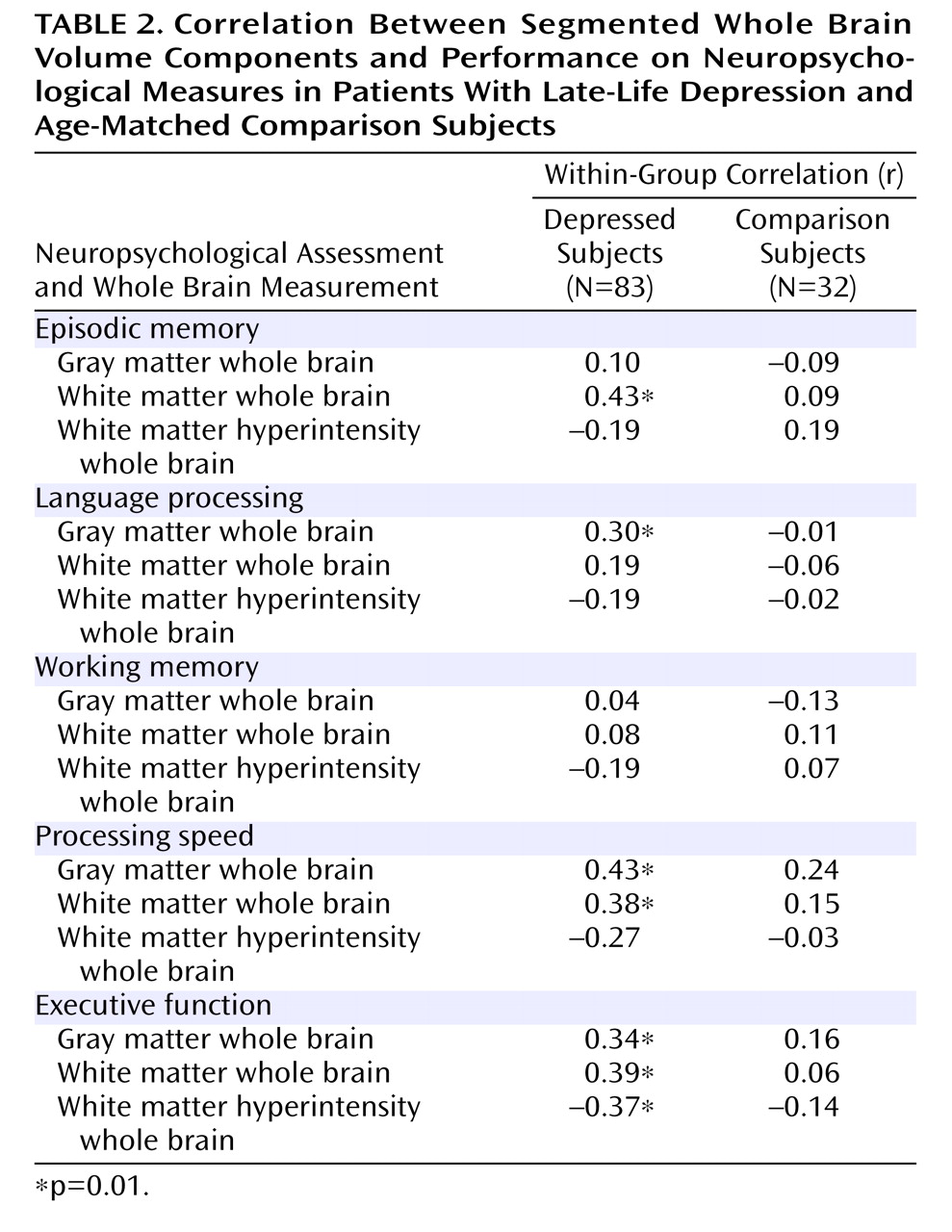
In addition, we examined the association between segmented whole brain volumetric measures and neuropsychological function. As shown in
Table 2, there were no significant correlations between any of the whole brain measures and neuropsychological function in comparison subjects. Among depressed patients, there were significant correlations of white matter hyperintensities and gray and white matter with neuropsychological function. All of these correlations maintained their significance when age and education were partialled out. Fisher’s r-to-z transformations indicated that the correlation between executive function and white matter hyperintensities was significantly stronger in depressed patients relative to comparison subjects (z=1.65, p=0.05).
Discussion
This study adds to the understanding of the pathophysiology of late-life depression in several ways. We found increased white matter hyperintensity burden in patients with late-life depression relative to comparison subjects in specific brain regions, despite no differences in demographic variables, vascular risk factor scores, or whole brain gray matter, white matter, or white matter hyperintensity volumes. This supports the regional specificity of hyperintensities in depression. The finding
(1 –
4) of increased overall volumes of white matter hyperintensities in late-life depressed subjects relative to comparison subjects in studies that did not match depressed and comparison subjects on vascular risk factors may indicate that more vascular events occur in depression. Depression has been associated with increased rates of cardiovascular illness
(23), diabetes mellitus
(24), smoking, and hypertension
(25) . Thus, given that depressed subjects have increased rates of multiple factors comprising the overall vascular risk profile, it is important to control for vascular risk factors in comparing depressed and comparison subjects for white matter hyperintensities.
Second, we suggest that in order to optimize anatomical localization, it is important to utilize segmentation methodology that allows localization of white matter hyperintensities in atlas space. Current scoring techniques have variable interrater reliability
(26) and homogenize white matter hyperintensity characteristics by assigning a general score for size and appearance in order to yield a global index of lesion load without spatial localization. In this study, we used an automated segmentation procedure that was reproducible, quantitatively validated
(13), and precisely localized lesions in atlas space.
Third, using this technique we identified significant differences in white matter hyperintensity burden in specific brain regions. These lesions were located in deep white matter underlying cortical regions critical for executive function and emotional processing, and lesion volumes correlated with executive function in three regions. A potential interpretation of these findings is that regionally specific hyperintensities may increase susceptibility to depression and may be expressed functionally as cognitive impairment. An extensive literature has described the importance of cognitive control and disturbances thereof in late-life depression
(27) . Further, of particular importance to emotional disturbances in depression are the white matter connections of the cingulate cortex, insula, and amygdala that were interrupted in the present study.
Lesions in the majority of fasciculi interrupted by hyperintensities in this study have been associated with specific cognitive impairment syndromes. The superior longitudinal fasciculus and fronto-occipital fasciculus are critical for cognitive control. A recent comprehensive compendium of white matter tracts in nonhuman primates and humans described these pathways in detail
(28) . The primary long association fibers connecting the frontal cortex are the three subdivisions of the superior longitudinal fasciculus: components I, II, and III
(28) . In the frontal lobe, superior longitudinal fasciculus 1 fibers project to the supplementary motor area and dorsal Brodmann’s areas 6 and 9 and convey information from these areas back to the parietal lobe cortices and probably into the precuneus. Superior longitudinal fasciculus 2 connections terminate in Brodmann’s areas 6, 8, 9/46, and 44, and superior longitudinal fasciculus 3 fibers terminate in ventral Brodmann’s areas 6 and 44.
Four regions of white matter hyperintensity lesions (right superior longitudinal fasciculus 1/fronto-occipital fasciculus; right superior longitudinal fasciculus 2/3; second region of right superior longitudinal fasciculus 2/3; left superior longitudinal fasciculus 2/3) were in white matter underling the dorsolateral prefrontal cortex (Brodmann’s area 9/46), and another region was located in the right extreme capsule of the basal ganglia, all interrupting the dorsolateral prefrontal circuit, namely connections between the basal ganglia and dorsolateral prefrontal cortex gray matter that are important for performance on tests of executive function
(27,
29) . This circuit projects from Brodmann’s area 9/46 to the dorsolateral caudate, then to the lateral mediodorsal globus pallidus and rostrolateral substantia nigra. The basal ganglia projects to the ventral anterior and mediodorsal thalamus and back to the dorsolateral prefrontal cortex, completing the loop
(30) . A large literature supports the association of the dorsolateral prefrontal cortex lesions with impairments in executive function
(31,
32) . Such lesions are found with greater frequency in major depression
(26) and in some studies were associated with worse treatment outcome
(33) . Consistent with this prior work, in our study, white matter hyperintensity lesion burden in the superior longitudinal fasciculus 1 and superior longitudinal fasciculus divisions 2 and 3 regions and right extreme capsule was correlated with executive function in depressed subjects, although only the left superior longitudinal fasciculus divisions 2 and 3 correlation survived Bonferroni correction.
Many of the long fasciculi such as the superior longitudinal fasciculus 1, 2, and 3 have connections to Brodmann’s area 6. While traditionally thought of as a motor region, more recent work has shown Brodmann’s area 6 to be involved in cognitive tasks
(34), including verbal operations
(35) . Intriguingly, there were significant correlations between white matter hyperintensities in the left superior longitudinal fasciculus divisions 2 and 3 and executive function in depressed but not comparison subjects in our study. The extreme capsule is important for language function linking Wernicke’s area with Broca’s area (Brodmann’s areas 44 and 45), and stimulation of the cortical areas to which it links results in speech arrest
(36) . Our depressed subjects had a significant relationship between lesions in this area and language function.
Several of the lesions in the present study interrupt pathways that are important in emotional circuitry. The cingulate gyrus has been implicated in many studies of emotional function. The major long association fibers that have been demonstrated emerging from the caudal cingulate gyrus include fibers in the dorsal part of the superior longitudinal fasciculus 1; fibers directed to the frontal lobe in the fronto-occipital fasciculus; and fibers in the cingulum bundle, uncinate fasciculus, and extreme capsule
(28) . The uncinate fasciculus is a ventral limbic pathway connecting temporal regions with medial and orbital frontal cortices. A contingent of fibers within the uncinate fasciculus leads from the parahippocampal region to the orbitofrontal cortex. Some fibers terminate in medial forebrain areas, and others terminate in Brodmann’s areas 13, 47/12, 11, and 10, all areas involved in emotional circuitry. Some fibers from the posterior parahippocampal gyrus also terminate in the rostral cingulate cortex (Brodmann’s area 24).
The amygdala connections interrupted by lesions of the uncinate fasciculus have important implications in disorders of emotion processing
(29,
37,
38) . White matter hyperintensity lesions in the inferior longitudinal fasciculus adjacent to the parahippocampal gyrus interrupt connections between V4 in the occipital cortex to the medial temporal structures, amygdala, hippocampus, and parahippocampus
(39) . The amygdala is known to have neuromodulatory effects on the extrastriate visual cortex
(40) . Thus, in depression it may be that the affective valence signals are not normally transmitted.
In addition to the impact of specific lesions, there were also significant correlations between segmented whole brain volumes and neuropsychological function in depressed subjects that were not found in comparison subjects, in particular, a significant correlation of whole brain white matter hyperintensities with executive function. Other studies have also found associations between white matter hyperintensity volumes and function, including
(41) an association between white matter hyperintensities and global reductions in cortical metabolic rates. Whole brain white matter was correlated with executive function, episodic memory, and processing speed in depressed subjects but not comparison subjects. Whole brain gray matter was correlated with processing speed and showed tendencies toward correlation with executive function and language in depressed patients but not comparison subjects. These patterns suggest stronger relationships between whole brain white and gray matter volumes and neuropsychological performance in depressed patients relative to comparison subjects. However, this interpretation should be moderated by the greater power to detect relationships in depressed patients relative to comparison subjects because of their larger numbers. Further, it is somewhat surprising that we did not find a relationship between neuropsychological performance and whole brain measures of white matter hyperintensity burden in comparison subjects, since several previous studies
(42,
43), but not all
(44), have found such relationships. In the seven regions showing group differences in white matter hyperintensities, we did find significantly less white matter hyperintensity variance in comparison subjects relative to depressed subjects, potentially contributing to an absence of correlations with neuropsychological variables in comparison subjects. However, we found no group differences in variance for any of the neuropsychological measures themselves, or for any of the whole brain measures. Thus, we argue that in addition to the interruption of specific neuroanatomical pathways by white matter hyperintensities contributing to the development of late-life depression there is an interaction between overall white matter hyperintensity burden and gray and white matter loss in depressed subjects producing increased neuropsychological deficits. The mechanism for this interaction is unclear and worthy of further investigation.