Despite recent public health efforts, regular smoking is still the most frequent attributable cause of premature disability and death in Western societies
(1) . Nicotine as the primary addictive compound of tobacco smoke binds at nicotinergic acetylcholine receptors located at neurons facilitating γ-aminobutyric acid (GABA) and dopamine transmission within different brain regions, such as the prefrontal cortex, thalamus, ventral tegmental area, and nucleus accumbens
(2,
3) . Like other drugs of abuse, nicotine provokes a sustained dopamine release in the nucleus accumbens, as shown by rat microdialysis experiments, that is important for the development of substance dependence
(4) .
Positron emission tomography (PET) provides a powerful tool to monitor pre- and postsynaptic changes within the dopamine system in the living brain
(5) . Consumption of a cigarette during a break from a [
11 C]raclopride PET scan results in a prominent decrease of dopamine 2 (D
2 ) receptor availability in the left ventral striatum, potentially representing a nicotine-triggered dopamine release
(6) . In addition, striatal uptake of the dopamine precursor [
18 F]fluorodopa was 16%–29% higher among regular smokers than among nonsmoking comparison subjects
(7) . However, the nicotine-induced displacement of [
11 C]raclopride by endogenous dopamine could not be confirmed in another study
(8) .
Given the provisional evidence that short-term use of nicotine releases dopamine within the ventral striatum in subjects who smoke for pleasure on a nonregular basis, it remains unclear whether prolonged nicotine intake and sustained dopamine release in dependent smokers might trigger hypofunctionality of the mesolimbic dopamine system. A significantly lower D
1 binding potential in the ventral striatum and caudate of chronic smokers than in nonsmoking subjects implies such a conclusion
(9) . Significantly lower striatal D
2 receptor availability, another marker for a hypofunctional dopaminergic state, has been observed in patients with alcohol
(10), heroin
(11), cocaine
(12), and methamphetamine
(13) dependence. In the case of alcohol-dependent subjects, the lower D
2 receptor availability persisted even 6 weeks after alcohol detoxification
(10,
14,
15), and it only slowly recovered several weeks after detoxification
(10,
14) . Given the high relapse rates during early abstinence, the low striatal D
2 receptor availability, either as a cause or a consequence of prolonged substance consumption, might therefore trigger relapse into compulsive substance consumption. This hypothesis is supported by the finding of an inverse correlation of striatal D
2 receptor availability with alcohol craving and cue-induced activation of several brain regions, such as the medial prefrontal cortex and anterior cingulate cortex, in a group of recently detoxified alcohol-dependent patients
(15) .
In the present study, D
2 receptor availability was measured in nicotine-dependent smokers at two time points (in consumption and withdrawal conditions) and in never-smoking subjects, by means of the high-affinity D
2 /D
3 receptor ligand [
18 F]fallypride. In contrast to [
11 C]raclopride, [
18 F]fallypride has a very high affinity for D
2 /D
3 receptors that allows the quantification of extrastriatal, e.g., fronto- or temporocortical, D
2 receptors
(16,
17) . We hypothesized that nicotine-dependent smokers would display less availability of striatal and potentially extrastriatal D
2 /D
3 receptors than would the never-smoking subjects, i.e., differences similar to those observed in patients suffering from alcohol, amphetamine, cocaine, or opioid dependence. Furthermore, if the lower availability would rely only on the nicotine-induced displacement of the radioligand by endogenous dopamine, an increase in D
2 receptor availability should be observed during nicotine withdrawal.
Discussion
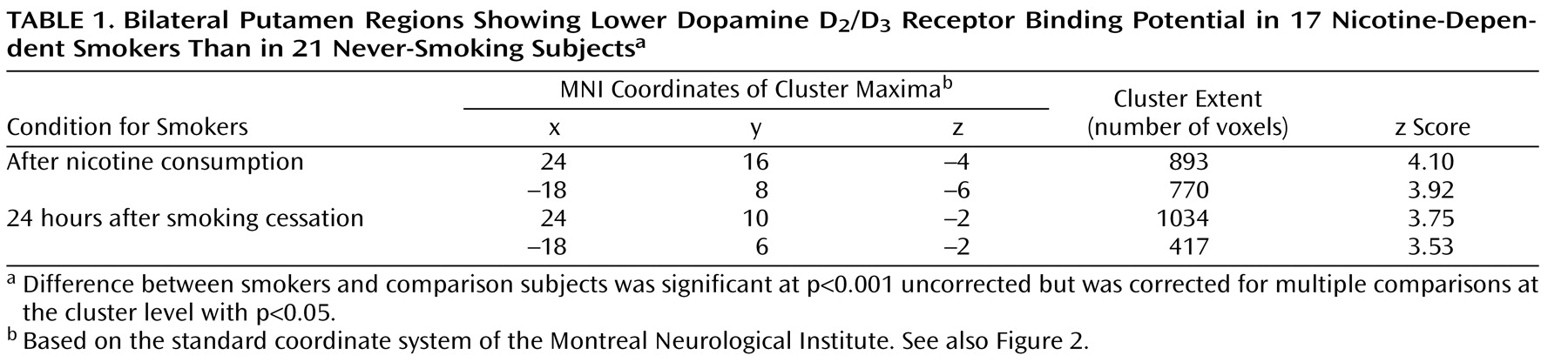
A major finding of the present study was the significantly lower D
2 /D
3 receptor availability in the bilateral putamen covering a major part of the dorsal striatum in heavy-smoking nicotine-dependent patients. Similarly low dorsal striatal D
2 receptor availability has been measured in patients dependent on alcohol
(10), heroin
(11), cocaine
(12), and amphetamine
(13) during either consumption or early abstinence. However, a sustained deficit in D
2 /D
3 receptor availability has been shown even when synaptic dopamine levels have returned to normal after stimulation
(24 –
26) . Thus, the low D
2 /D
3 receptor availability after 24 hours of abstinence might not actually reflect a low level of synaptic dopamine at that time point but could result from sustained low receptor availability due to nicotine-induced dopamine release. Nevertheless, low D
2 /D
3 receptor availability either during consumption or during early abstinence has been viewed as a characteristic endophenotype defining substance dependence and potentially triggering relapse risk
(5) . If confirmed among nicotine-dependent patients several weeks after smoking cessation, the low striatal D
2 /D
3 receptor availability observed in our study might explain the frequently experienced difficulties in becoming or staying abstinent from nicotine.
However, these considerations have to be limited to heavy-smoking nicotine-dependent subjects, such as those investigated in our study. Striatal dopamine release in response to acute tobacco/nicotine consumption has been substantiated by a number of human and primate experiments using [
11 C]raclopride
(6,
27,
28) . The magnitude of the reported alteration in D
2 /D
3 receptor availability varies substantially across these studies, but it seems to be smaller than that seen with amphetamine administration
(28,
29) . However, these studies are unable to answer the question of whether the low striatal D
2 receptor availability is a result of nicotine-induced dopamine release over time or whether it is a trait increasing the liability for nicotine dependence, as none of these studies investigated nonaddicted smokers, addicted smokers, and never-smokers simultaneously
(30) . Nonaddicted smokers might even display a high availability of striatal D
2 receptors that protects them against nicotine dependence, as shown for the nonaddicted first-degree relatives of alcohol-dependent patients
(31) .
In our study, a low availability of striatal D
2 /D
3 receptors among nicotine-dependent smokers was found in large areas of the bilateral putamen covering different parts of the striatum, such as the limbic, associative, and sensorimotor striatum
(32) . Lesion experiments in rodents have implicated functional subdivisions in the midbrain dopamine system, with the nucleus accumbens shell mediating psychostimulant drug effects and the nucleus accumbens core being important for priming cue-induced drug craving
(33,
34) . Functional subdivisions have also been proposed for the human striatum
(32) ; however, as in our study, low [
11 C]raclopride binding among cocaine-dependent subjects was observed within all three parts of the striatum, i.e., the limbic, associative, and sensorimotor striatum
(35) . Thus, nicotine-induced alterations are not limited to the regions mediating unconscious drug seeking; presumably they also affect neurobiological regions initiating drug-stimuli conditioning as well as the psychomotor effects of nicotine stimuli
(5) .
In our study, overnight nicotine withdrawal did not alter D
2 /D
3 receptor availability among the nicotine-dependent subjects. As mentioned earlier, [
18 F]fallypride binding 24 hours after smoking cessation still might have remained low because of the effects of nicotine-induced dopamine release
(24 –
26) . Alternatively, the low striatal D
2 /D
3 receptor availability after overnight withdrawal might be maintained by mechanisms that go beyond direct competition with endogenous dopamine.
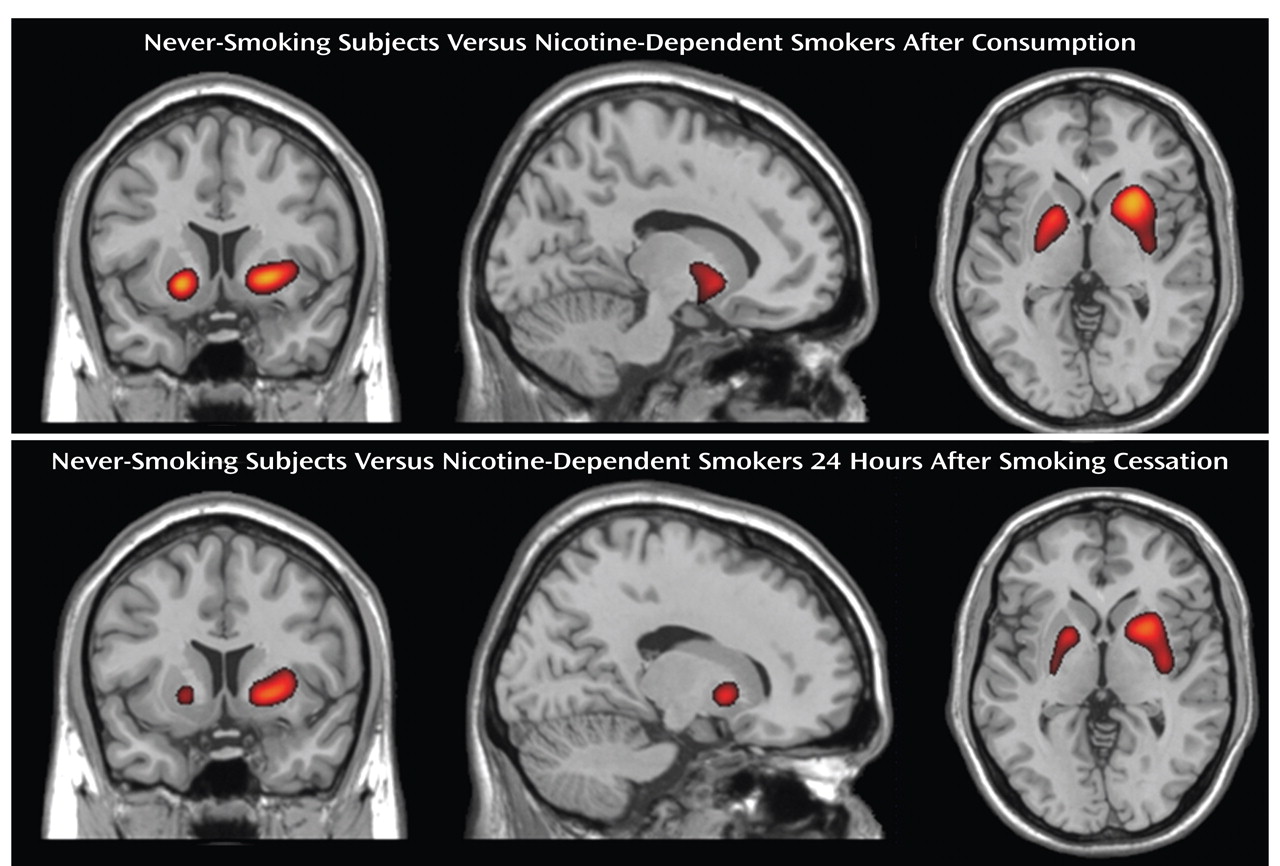
In contrast to the observed differences in striatal D
2 /D
3 receptor availability between the nicotine-dependent smokers and nonsmoking subjects, no significant differences in extrastriatal D
2 /D
3 receptor availability could be detected between the two groups (
Figure 2 ). Given the substantially lower [
18 F]fallypride binding potentials in the extrastriatal regions as compared to the striatum in healthy subjects studied by Siessmeier et al.
(16) (mean binding potential in thalamus, 1.9–2.2, mean in striatum, 21.7–23.3), this could have resulted from a relatively large intersubject variability in extrastriatal D
2 receptor availability hampering the measurement of a statistically significant difference. Besides, there might not be any extrastriatal difference in D
2 /D
3 receptor availability if the low striatal D
2 availability results only from the prolonged nicotine-induced dopamine release that is most prominent in the nucleus accumbens, as suggested by experiments using animal models
(33,
34,
36) .
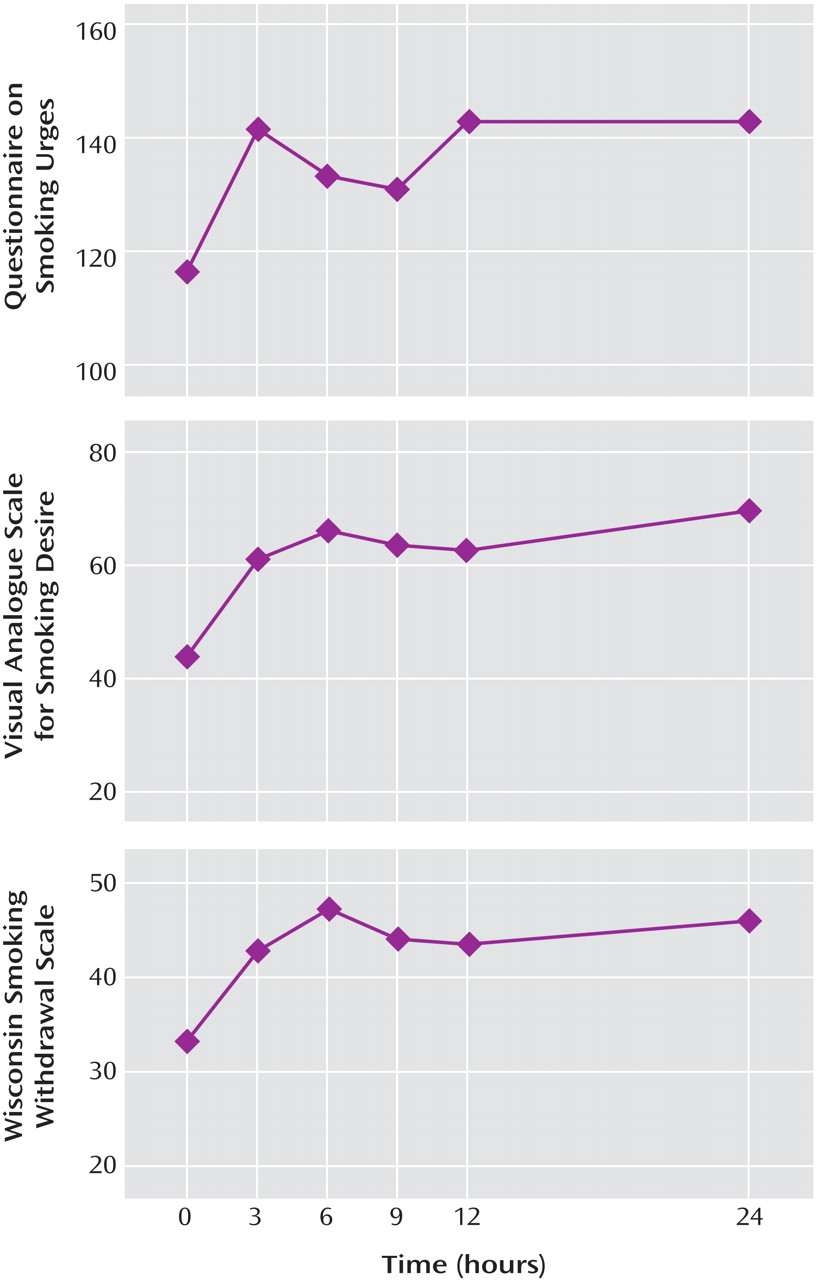
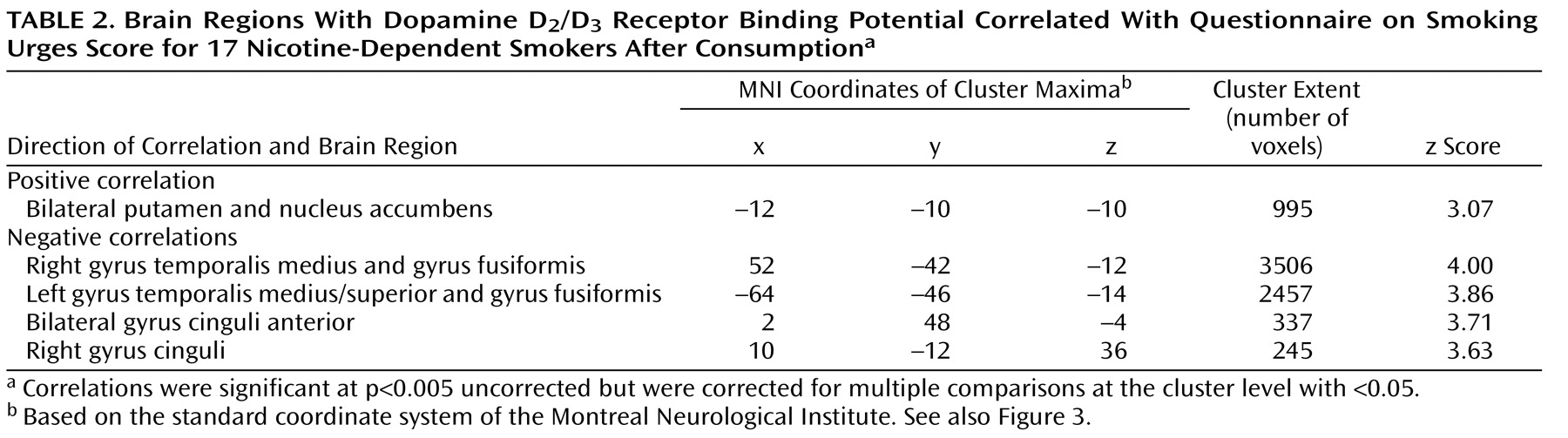
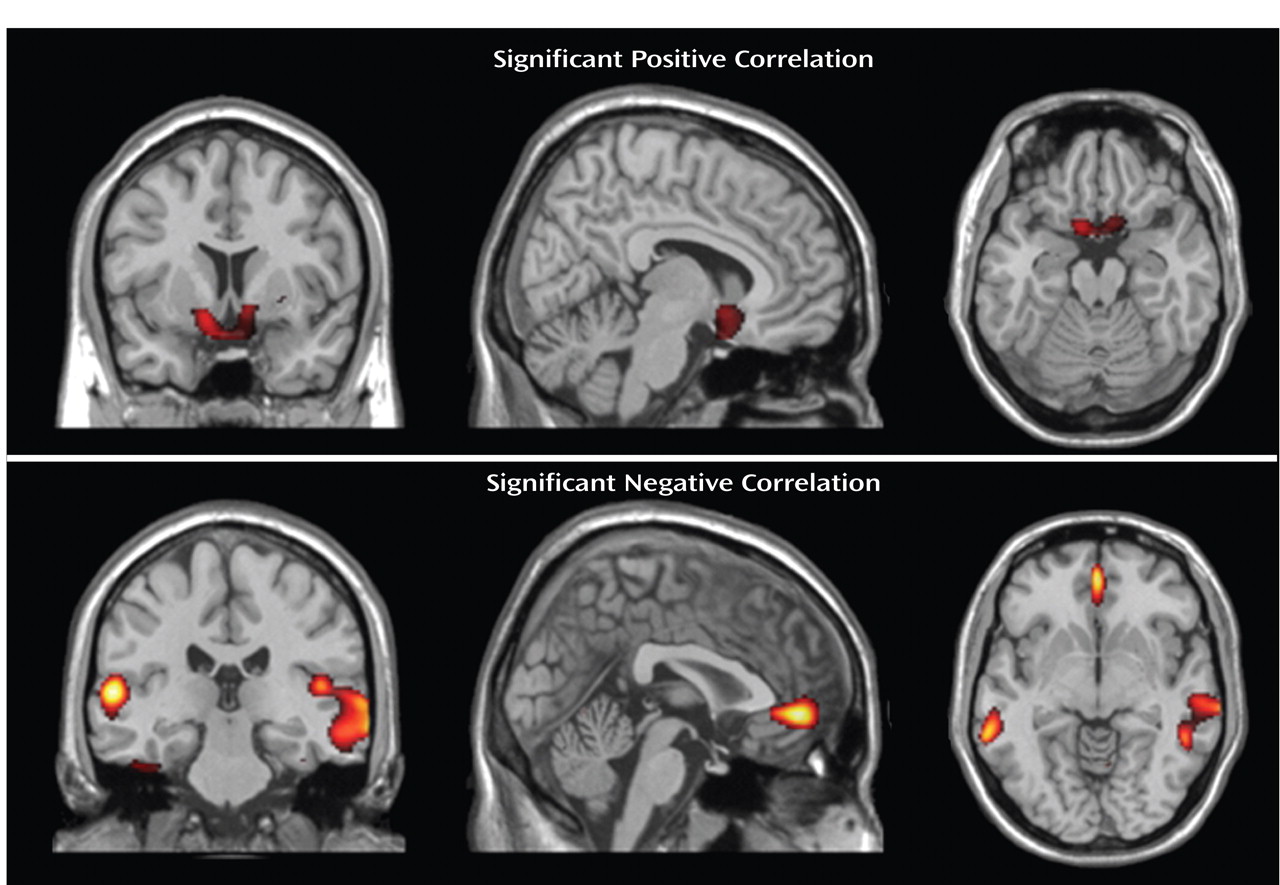
Contrary to our primary expectations, nicotine craving as measured with the Questionnaire on Smoking Urges positively correlated with D
2 /D
3 receptor availability in the ventral striatum. Given the likelihood that higher D
2 /D
3 receptor availability during consumption is the indirect measure of lower striatal dopamine levels, one likely explanation is that a stronger desire to smoke results from impaired nicotine-induced dopamine release. This explanation is appealing, as behavioral experiments in animal models have repeatedly demonstrated the importance of the mesolimbic dopamine system for attributing incentive salience to drugs of abuse, finally leading into compulsive drug taking and drug wanting
(37) . Alternatively, the positive correlation between smoking desire and D
2 /D
3 receptor availability could be a result of a sensitization process that might have occurred after numerous years of smoking. Consistent with that hypothesis, an increased sensitivity to the D
2 receptor agonist quinpirole triggering cocaine reinstatement has been described among withdrawn rats with high previous cocaine intake
(38) . It should be noted that some previous studies found no association
(35) or a negative relationship
(15,
39) between drug craving intensity and striatal D
2 receptor availability. The observed discrepancies between the studies may be explained by different substances investigated, variations in study protocols, or the perceived opportunity of drug use in the case of nicotine
(40) .
In contrast to the positive correlation between striatal D
2 /D
3 receptor availability and craving, we observed a negative correlation between craving and the D
2 /D
3 receptor availability in the anterior cingulate cortex, inferior temporal cortex, and fusiform cortex. This is of particular interest as cue-induced nicotine craving has been repeatedly shown to activate the anterior cingulate cortex
(30) . Our data also support findings from experimental studies on rats that have emphasized the role of the prefrontal dopamine system for the reinstatement of drug-seeking behavior
(41) . If confirmed by independent investigations, nicotine craving might therefore be maintained by a spatial shift in D
2 /D
3 receptor availability, with a relatively high proportion of available D
2 /D
3 receptors in the striatum and lower proportions of D
2 /D
3 receptors available in the anterior cingulate cortex and inferior temporal cortex that may lead to disinhibition of frontocortical glutamatergic neurons
(42) .
In this study, nicotine-dependent heavy smokers displayed lower availability of striatal D 2 /D 3 receptors than did subjects who had never smoked. Contrary to our primary hypothesis, we did not find a negative correlation between nicotine craving and ventral striatal D 2 receptor availability. Our results imply, rather, that nicotine craving in a condition of average consumption is maintained by a region-specific shift in D 2 /D 3 receptor availability, with greater availability within the striatum and less availability within the anterior cingulate cortex and inferior temporal cortex.