During the last decades, our understanding of the development and maintenance of anxiety disorders has improved by advances in the analysis of learning mechanisms such as Pavlovian conditioning
(1) . Pavlovian conditioning is a universal learning process present in humans and animals and is characterized by the acquisition of a conditioned response to an initially neutral stimulus, which becomes a conditioned stimulus (CS) by its association with a biologically relevant stimulus. The conditioned response is often, although not always, similar to the unconditioned response, which is the initial reaction to the unconditioned stimulus. In posttraumatic stress disorder (PTSD), a prototypical anxiety disorder, the trauma can be considered an unconditioned stimulus, and the continued fear response in PTSD patients can be considered a conditioned response
(2,
3) . PTSD has in fact been associated with enhanced acquisition and slower extinction of fear responses
(4,
5) . Furthermore, the observed enhanced peripheral
(6), electrophysiological
(7,
8), and hemodynamic responses to trauma-related cues in PTSD patients
(9) have been suggested to result from conditioning to the trauma. Yet, since the trauma is no longer present and thus the expectation of danger signaled by the conditioned stimulus is not met, the conditioned fear response should extinguish. This is, however, not the case in PTSD, in which fear responses are maintained many years after the original trauma.
Second-order conditioning refers to the well-documented fact that the conditioned stimulus can acquire the properties of the unconditioned stimulus and elicits conditioned responses to new neutral stimuli that are associated with it
(10) . In PTSD, trauma-related cues might over time come to serve as unconditioned stimuli and thus acquire the properties of the original trauma. Stimulus generalization with an enlarged fear-related memory network might ensue
(11), and the extinction of conditioned responses may be impaired by the involuntary re-experiencing of the traumatic event.
Consistent with conditioning theories of PTSD, several studies have investigated Pavlovian conditioning in PTSD patients using trauma-irrelevant aversive cues (e.g., painful electric stimulation) as unconditioned stimuli and revealed enhanced acquisition
(5) and slower extinction of the conditioned response in PTSD patients
(5,
12), as well as generalization of fear and sensitization by stress
(13) . Increased amygdala activation during fear acquisition and decreased anterior cingulate activity during extinction have also been reported in PTSD patients
(14), suggesting insufficient inhibitory inputs from the medial prefrontal cortex to the amygdala as a maintaining factor in PTSD.
Since higher-order conditioning phenomena appear to be highly relevant to anxiety disorders
(10), we investigated second-order conditioning in PTSD patients, using a trauma-specific cue as an unconditioned stimulus and neutral graphics as conditioned stimuli. Consistent with previous conditioning studies in other patient groups
(15,
16), we assessed self-report, electrodermal, heart rate, and startle measures. Additionally, the P300 and terminal contingent negative variation of the electroencephalogram (EEG) were used as indicators of brain processing during Pavlovian conditioning
(15,
17 –
19) . We predicted that PTSD patients would exhibit second-order conditioning as evidenced by an enhanced acquisition and slower extinction of the conditioned response on all levels.
Method
Participants
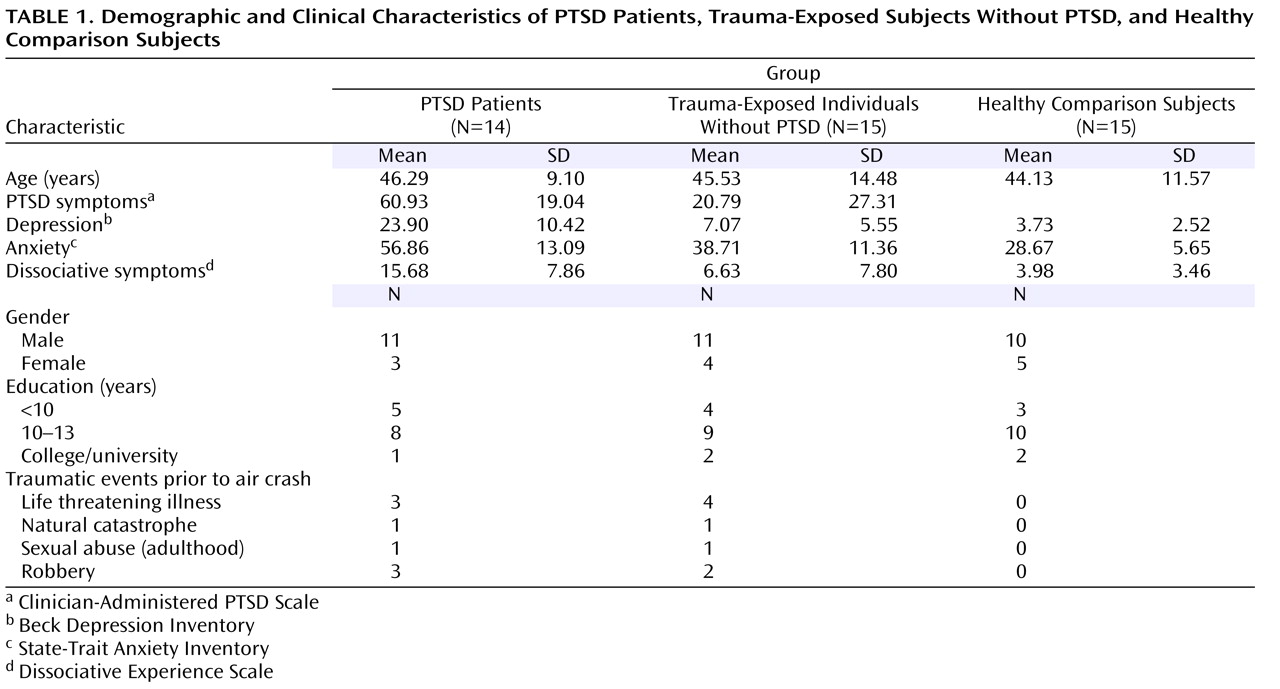
Twenty-nine trauma-exposed subjects and 15 age- and sex-matched healthy comparison subjects participated in the study (
Table 1 ). Healthy comparison subjects were recruited through advertisements in newspapers, and trauma-exposed subjects were recruited exclusively among survivors of the air show disaster in Ramstein, Germany in 1988, resulting in a homogenous cohort with respect to the type of trauma and the time span between the traumatic event and the experiment. All trauma-exposed subjects experienced the traumatic event at a similar distance from the airplane crash, as verified by questionnaires and interviews about geographical landmarks of the air base, and thus they encountered a similar degree of danger arising from the crash. Some trauma-exposed subjects experienced other traumatic events prior to the air crash, with no significant differences between the two trauma-exposed groups (
Table 1 ). Healthy comparison subjects had never experienced a traumatic event.
All trauma-exposed subjects fulfilled the trauma criteria of DSM-IV-TR
(20) . Based on the Clinician-Administered PTSD Scale
(21), 14 trauma-exposed subjects were diagnosed with current PTSD, and 15 trauma-exposed subjects had neither current nor lifetime PTSD. The two trauma-exposed groups were not significantly different with respect to their injuries sustained in the traumatic air crash event. Two PTSD patients and one trauma-exposed subject without PTSD reported a major depressive episode in the past. None of the study participants had ever had a neurological disorder or fulfilled the criteria for current affective disorders, current alcohol/drug dependence or abuse, or current or lifetime psychotic symptoms as verified by the Structured Clinical Interview for DSM-IV
(22) . Healthy comparison subjects had never met any criterion for a DSM-IV-TR disorder. The study was approved by the local ethics committee. All participants signed informed consent.
Experimental Procedures
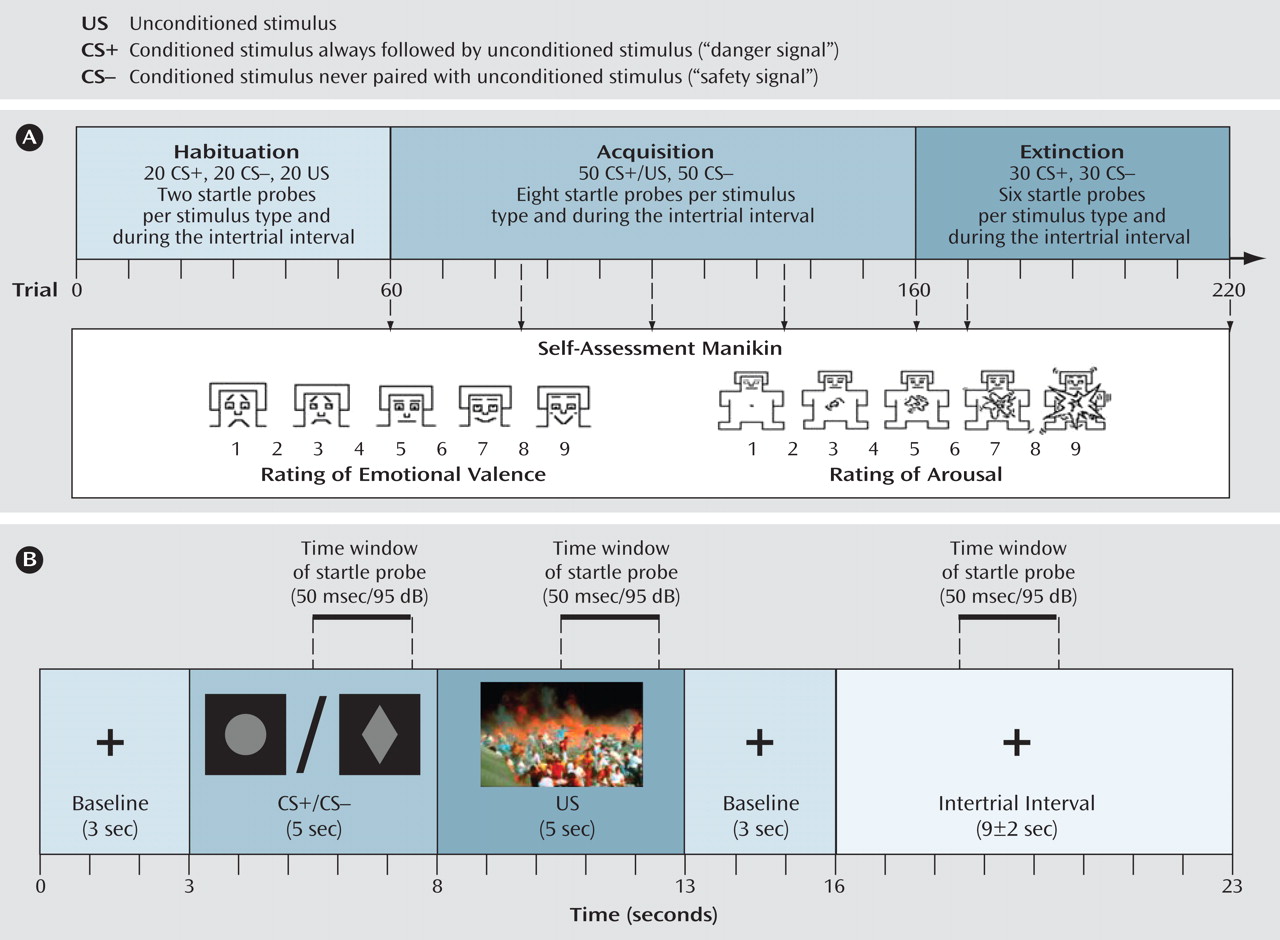
We used a differential delay-conditioning paradigm in which a trauma-specific visual cue served as an unconditioned stimulus (see
Figure 1 in the data supplement accompanying the online version of this article). The trauma-specific cue was pretested in an independent cohort of healthy comparison subjects (N=93) and study participants using the Self-Assessment Manikin
(23), a nonverbal scale, which assesses valence and arousal of a stimulus and provides scores from 1 (very unpleasant/not arousing) to 9 (very pleasant/very arousing). Additionally, trauma-exposed subjects completed a visual analogue scale asking for the personal relevance of the picture for their trauma (0=not relevant to 100=extremely relevant). Twenty pictures depicting the air crash and 20 neutral pictures from the International Affective Picture System
(24) were presented in the pretest. The air crash picture with the highest average relevance for trauma-exposed participants (95.5 [SD=5.82]) and the most aversive and arousing ratings for healthy comparison subjects (valence:1.88 [SD=1.30]; arousal: 6.84 [SD=1.88]) was chosen as the unconditioned stimulus.
Figure 1 displays the structure of the differential delay conditioning paradigm. One neutral stimulus was immediately and always followed by an unconditioned stimulus, representing a danger signal (CS+), whereas a second neutral stimulus was never followed by the unconditioned stimulus, thereby representing a safety signal (CS−). In this study, two graphic representations (circle, rhombus) were used as the CS+ and CS−. Stimulus order was pseudorandomized so that one stimulus type was not repeated more than three times in sequence.
Following the habituation, after every 25th, 50th, 75th, and 100th acquisition trial and the 10th and 60th extinction trial, we assessed emotional valence and arousal ratings using the Self-Assessment Manikin, and we assessed unconditioned stimulus-expectancy after conditioned stimulus presentation using a visual analogue scale with nine steps (1 [unconditioned stimulus, not at all expected] to 9 [unconditioned stimulus, 100% expected]). EEG from 30 channels and skin conductance responses, heart rate, and electromyogram (EMG) at the m. orbicularis oculi (startle response) were recorded during the experiment.
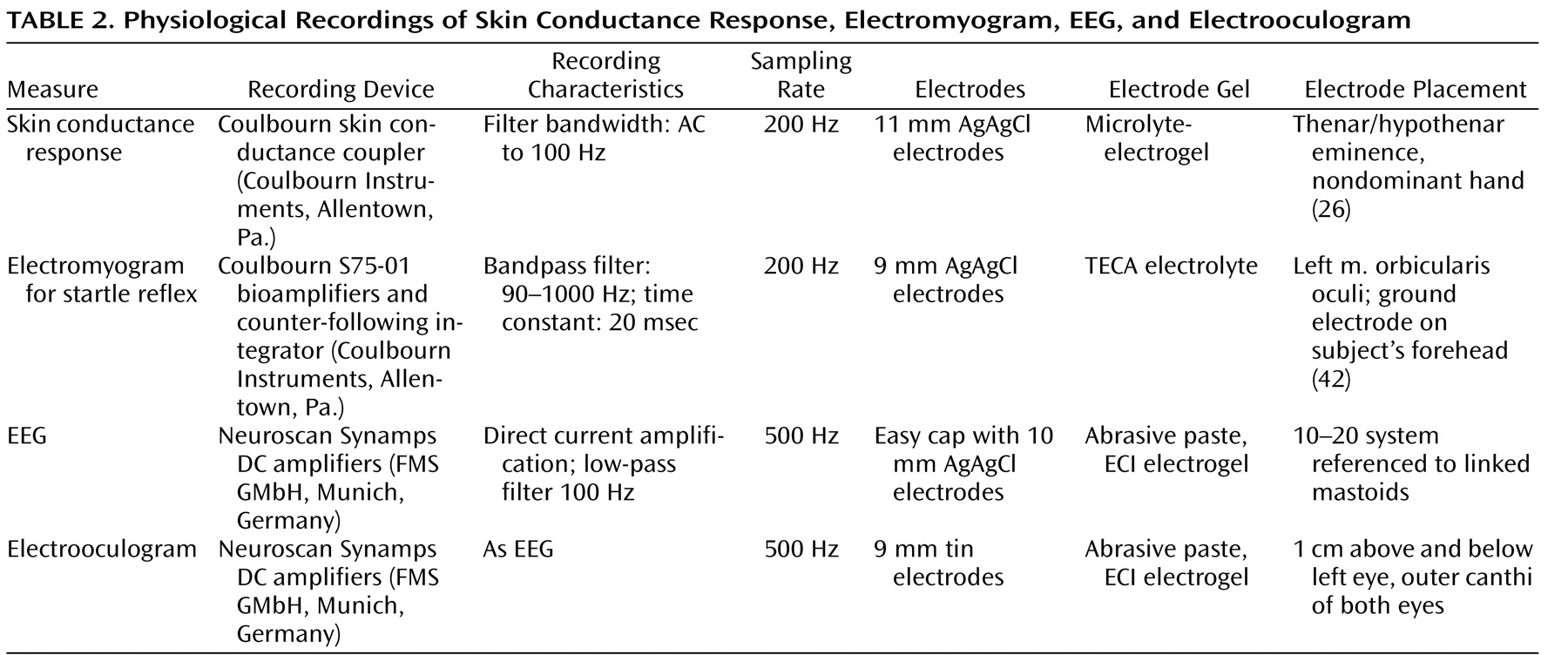
Physiological Recordings
All physiological data were collected continuously and digitally sampled. Detailed description of the physiological recording devices and characteristics are displayed in
Table 2 .
Data Reduction and Analysis
Trials with startle probes were excluded from the analyses of all data other than startle. The EEG data were segmented separately for each stimulus category (CS+, CS−, unconditioned stimulus) and electrode using BrainVision Analyzer 1.03 (Brain Products GmbH, Munich, Germany), and eye movement corrections were performed
(25) . Prestimulus baselines were 100 msec for P300 and 500 msec for terminal contingent negative variation. No bad trials needed to be excluded for habituation. An average of 12.36 trials (SD=4.96) were excluded from analyses for acquisition, and 6.28 trials (SD=3.11) were excluded for extinction, with no significant group differences. The remaining trials were averaged and filtered with a low-pass filter of 6-Hz. The P300 peak amplitude was determined automatically between 280 and 600 msec post-stimulus onset. The terminal contingent negative variation was defined as the average amplitude of the 500 msec time window prior to stimulus onset.
For skin conductance responses, the maximum response in the time window from 1 to 4 seconds after stimulus onset
(26) was employed and normalized using a log (one positive skin conductance response) transformation. Amplitudes below 0.05 msec were classified as zero responses.
Heart rate change scores for the respective stimulus types were calculated by subtracting a 3-second baseline value from the 5-second conditioned/unconditioned stimulus presentation values. Since heart rate showed only the previously documented higher baseline levels in PTSD patients (F=3.83, df=2, 36, p<0.05) but no differential conditioning effects (c.f.,
16,
27 ), these data are not reported in this article.
The amplitude of the startle response was defined as the difference between the baseline and peak of the blink response occurring between 20 and 120 msec after stimulus onset. A lack of blink responses was scored with 0. An average of 1.68 zero trials (0.95) were excluded for acquisition and 1.12 trials (0.63) for extinction, with no significant group differences. To control for individual differences in overall blink magnitude, startle responses were standardized using a within-participant z score transformation
(28) .
As the electrodermal and startle response habituated significantly over time (both F values>5.50; both p values<0.01) and no interactions were observed with group differences (both F values<0.90; both p values>0.55), only the first half of each conditioning phase was included in the statistical analyses.
Statistical Analysis
Unless otherwise specified, group (PTSD, trauma-exposed without PTSD, healthy comparison)-by-conditioned stimulus type (CS+ or CS−)-by-phase (habituation, acquisition, extinction) repeated measures analyses of variance (ANOVAs) were performed. For self-report, the within-factor phase consisted of seven steps according to the assessment points. To compare the evaluation of the unconditioned stimulus with the conditioned stimuli, a factor conditioned-unconditioned stimulus type (CS+, CS−, unconditioned stimulus) was employed.
We analyzed event-related potentials from nine electrode sites (i.e., frontal [F3, Fz, F4], central [C3, Cz, C4], and parietal [P3, Pz, P4]) as also reported in other studies
(15) . This resulted in two additional within-subject factors: electrode position (frontal, central, posterior) and laterality (left, midline, right). Terminal contingent negative variation amplitudes were analyzed for acquisition and extinction.
We only report significant interactions with respect to group and conditioned stimulus type. In the case of significant interactions with the factor phase, ANOVAs for the individual conditioning phases were conducted. To verify an increased response to the CS+ relative to the CS− in each experimental group, we calculated within-group paired comparisons for the CS+/CS− difference with a Bonferroni-corrected significance level of 0.017.
Results
Unconditioned Response to the Trauma-Specific Stimulus
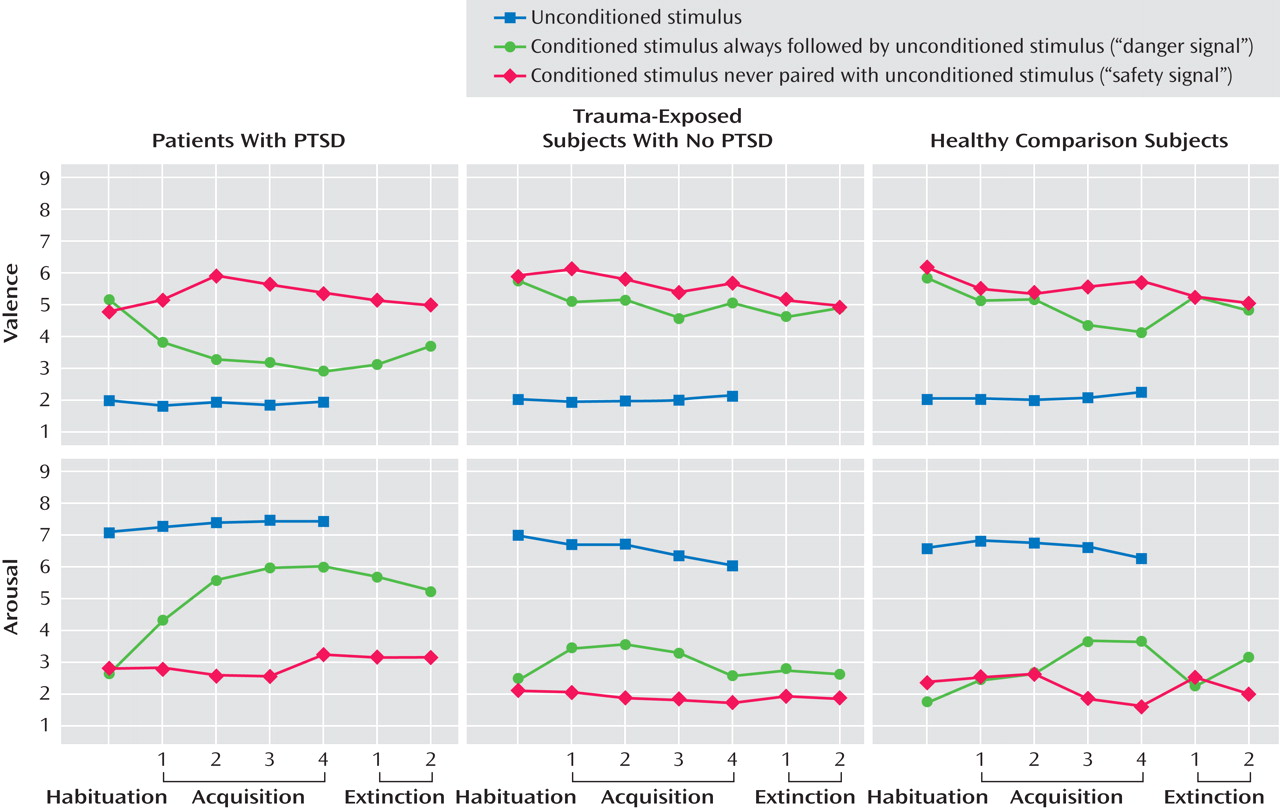
A significant unconditioned-conditioned stimulus type effect for valence and arousal indicated that all participants rated the unconditioned stimulus as more negative (F=100.34, df=2, 82; epsilon=0.74, p<0.001) and arousing (F=81.42, df=2, 82, p<0.001) than either the CS+ or CS− (
Figure 2 ). In all groups, skin conductance responses, startle responses, and P300 to the unconditioned stimulus were significantly higher relative to the conditioned stimuli (skin conductance responses: F=14.97, df=2, 78, p<0.001, epsilon=0.55; startle: F=3.38, df=2, 70, p<0.05; P300: F=33.56, df=4, 164, p<0.001).
Conditioned Responses
Descriptive and statistical values from paired comparisons for self-report, psychophysiological, and EEG data are displayed in Tables 1 and 2 in the data supplement accompanying the online version of this article.
Self-Report Measures
For valence ratings, significant conditioned stimulus type (F=17.85, df=1, 41, p<0.001), phase (F=5.68, df=6, 246, p<0.001), conditioned stimulus type-by-phase (F=7.43, df=6, 246, p<0.001), and conditioned stimulus type-by-phase-by-group effects (F=3.02, df=12, 246, p<0.01 [
Figure 2 ]) were observed. During acquisition, PTSD patients rated the CS+ significantly more negative (F=5.05, df=2, 41, p<0.05) compared with trauma-exposed subjects without PTSD (p=0.02) and healthy comparison subjects (p=0.05). During extinction, valence showed a significant group effect (F=4.88, df=2, 41, p<0.05), with PTSD patients rating the CS+ as more aversive than the CS− relative to healthy comparison subjects (p=0.02) but not compared with trauma-exposed subjects without PTSD. PTSD and trauma-exposed subjects without PTSD, but not healthy comparison subjects, differentiated significantly between the CS+ and CS− during acquisition, and only PTSD patients failed to extinguish the negative evaluation of the CS+.
For arousal, significant group (F=5.50, df=1, 41, p<0.01), conditioned stimulus type (F=20.96, df=1, 41, p<0.001), phase (F=3.96, df=6, 246, p<0.001), conditioned stimulus type-by-phase (F=8.54, df=6, 246, p<0.001), and conditioned stimulus type-by-phase-by-group effects (F=2.13, df=12, 246, p<0.05 [
Figure 1 ]) were found. PTSD patients were significantly more aroused than both other groups (PTSD patients versus trauma-exposed subjects without PTSD: p=0.02; PTSD patients versus healthy comparison subjects: p=0.02). During acquisition and extinction, PTSD patients rated the CS+ as significantly more arousing (acquisition: F=4.77, df=2, 41. p<0.05; extinction: F=7.68, df=2, 41, p<0.001) compared with trauma-exposed subjects without PTSD (acquisition: p=0.04; extinction: p=0.01) and healthy comparison subjects (acquisition p=0.03; extinction: p=0.004). All three groups showed significant CS+/CS− differentiation during acquisition, but only PTSD patients failed to extinguish.
The unconditioned stimulus-expectancy ratings revealed significant group (F=5.42, df=2, 41, p<0.01), conditioned stimulus type (F=194.16, df=1, 41, p<0.001), phase (F=56.75, df=6, 246, p<0.001), phase-by-conditioned stimulus type (F=66.04, df=6, 246, p<0.001), and phase-by-group (F=2.90, df=12, 246, p<0.05) effects. The latter indicated that during extinction, PTSD patients regarded both the CS+ (F=4.76, df=2, 41, p<0.05) and CS− (F=6.48, df=2, 41, p<0.01) as danger signals (PTSD patients versus trauma-exposed subjects without PTSD: CS+: p=0.03; CS−: p=0.03; PTSD patients versus healthy comparison subjects: CS+: p=0.03; CS−: p=0.004). All three groups learned the CS+/CS− unconditioned stimulus contingency correctly, but only PTSD patients failed to extinguish it.
Peripheral Measures
For skin conductance responses, a significant conditioned stimulus type effect (F=6.02, df=1, 39, p<0.05) indicated higher responses to the CS+ versus CS−. Only PTSD and trauma-exposed subjects without PTSD exhibited a differential electrodermal conditioned response as evidenced by within-group paired comparisons. A significant group-by-phase-by-conditioned stimulus type interaction (F=3.57, df=4, 78, p<0.05) indicated that only the PTSD group had skin conductance responses to the CS+ in the extinction phase (F=4.25, df=2, 39, p<0.05) and maintained the conditioned response.
A significant conditioned stimulus type-by-phase interaction (F=5.27, df=2, 70, p<0.01) revealed higher startle amplitudes for the CS+ versus CS− during acquisition for all groups. However, within-group paired comparisons indicated that this overall group effect derived mainly from a significant CS+/CS− differentiation in PTSD and trauma-exposed subjects without PTSD that was not present in healthy comparison subjects.
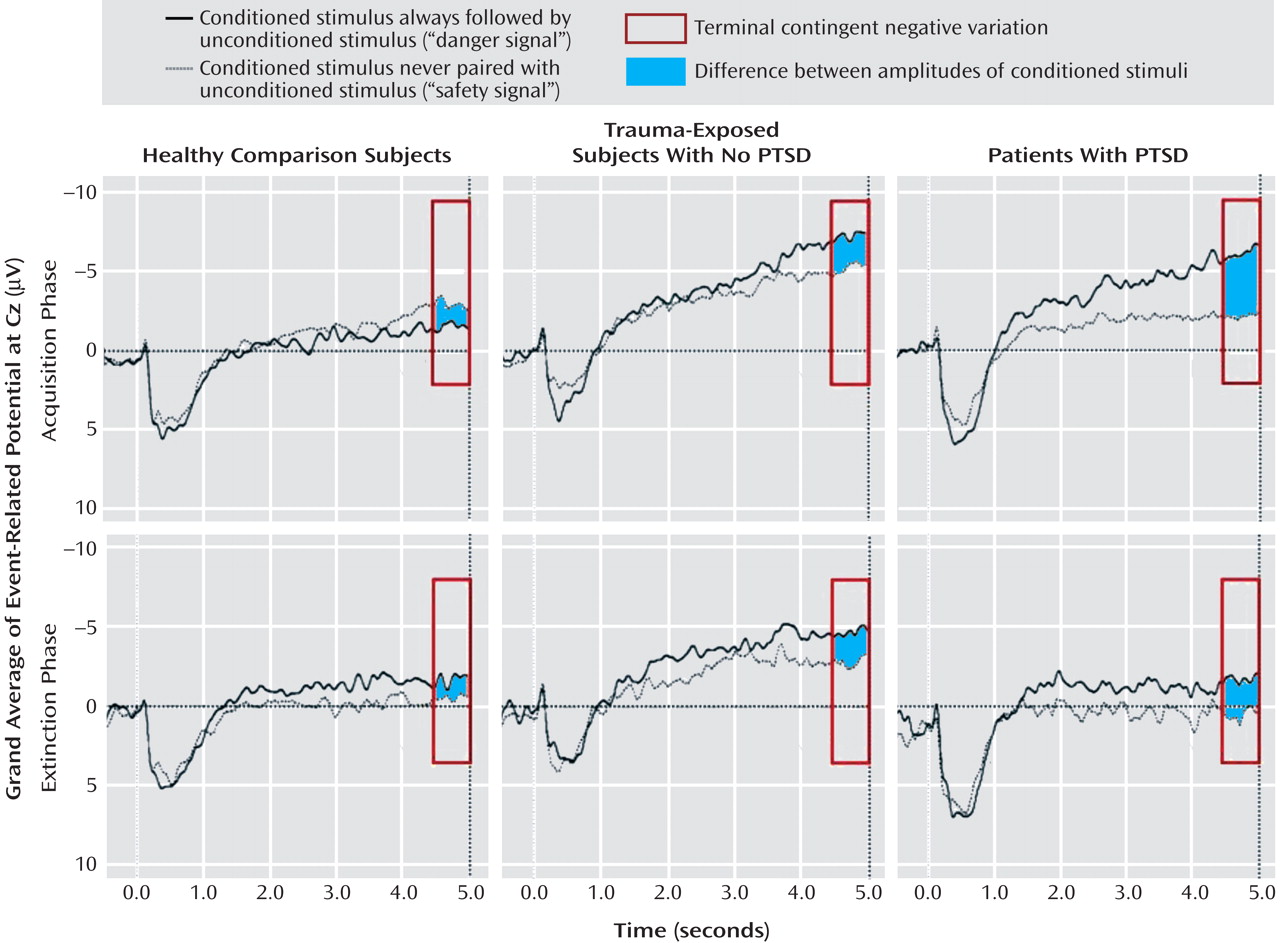
Event-Related Potential Components
Figure 3 displays EEG grand averages over Cz. For P300, significant conditioned stimulus type (F=6.62, df=1, 40, p<0.05) and conditioned stimulus type-by-phase-by-electrode position (F=15.03, df=2, 80, p<0.05) effects were found. P300 responses to the CS+ versus CS− were higher over central electrodes during acquisition (F=5.27, df=1, 40, p<0.05). Successful CS+/CS− differentiation as evident by within-group paired comparisons during acquisition was present in PTSD and trauma-exposed subjects without PTSD but not in healthy comparison subjects.
The terminal contingent negative variation showed a significant conditioned stimulus type effect (F=26.94, df=1, 40, p<0.001), with CS+ eliciting more negativity than CS− during acquisition and extinction. During acquisition, a significant conditioned stimulus type-by-phase-by-group effect (F=3.38, df=2, 40, p<0.05) indicated increased terminal contingent negative variation amplitudes to CS+ versus CS− in PTSD patients (F=5.32, df=2, 40, p<0.01). However, within-group paired comparisons revealed that both PTSD and trauma-exposed subjects without PTSD, but not healthy comparison subjects, showed terminal contingent negative variation-related CS+/CS− differentiation during acquisition. Although no significant ANOVA effects were found during extinction, within-group paired comparisons revealed enduring CS+/CS− differentiation during extinction in PTSD patients only.
Discussion
The present study revealed an enhanced conditionability to trauma-specific cues in traumatized persons with and without PTSD. Whereas in the acquisition phase both PTSD and trauma-exposed subjects without PTSD showed a differential conditioned response on all levels, healthy comparison subjects did not exhibit differential conditioning on any measure, except for self-reported arousal.
Only PTSD patients, but not trauma-exposed subjects without PTSD, showed a more negative and arousing evaluation of the CS+ and larger terminal contingent negative variation amplitudes to the CS+ relative to the CS−. The terminal contingent negative variation is well recognized as a marker of successful learning of a predictive relationship between two stimuli
(19,
29) and thus reflects a stronger central association between the CS+ as a danger signal and the unconditioned stimulus
(30) in PTSD patients. This provides first evidence for an increased expectancy toward potential threat stimuli on a central level in PTSD.
Most interesting was the failure to extinguish the conditioned response in PTSD patients when the trauma reminders were no longer present. This persistent conditioned response during extinction was reflected by a more negative and arousing evaluation of the CS+, increased skin conductance response amplitudes to the CS+, as well as a continued cerebral CS+/CS− differentiation. Even though skin conductance responses were generally diminished in late extinction, PTSD patients continued to show CS+/CS− differentiation. We also observed a general overestimation of unconditioned stimulus probability during the first half of the extinction phase in PTSD patients compared with both other groups, reflecting an overestimation of the danger value of both the CS+ and CS−. A similar effect has been reported in phobic patients
(31) who overestimate the unconditioned stimulus probability after a phobia-specific conditioned stimulus. In the present study, PTSD patients overestimated the occurrence of unconditioned stimulus when it was preceded by a neutral stimulus that actually served as safety signal, predicting the absence of the aversive trauma reminder. In accordance, previous studies
(12,
13) suggested that PTSD patients may have difficulties differentiating between relevant and irrelevant cues and learning to identify safety signals
(5) .
The finding of delayed extinction of a conditioned response has previously been demonstrated in first-order conditioning studies in PTSD
(5,
12) . A neuroimaging study of fear conditioning in PTSD patients
(14) reported an attenuated frontal response during extinction in patients relative to comparison subjects. Both animal and human studies suggest that intact ventromedial prefrontal functioning is crucial to successful extinction
(32), whereas a disruption of a frontolimbic network leads to a lack of inhibitory control over limbic structures and thus to impaired extinction of a previously acquired response
(33,
34) . Interestingly, homologous areas of the ventromedial prefrontal cortex have been shown to be functionally and morphologically altered in PTSD patients
(35 –
37) . Even if our data do not allow a direct confirmation of this hypothesis, they further underline the significance of altered extinction processes in PTSD.
The group differences in conditionability and resistance to extinction shown in the present study cannot be attributed to differential initial reactions to the trauma-specific unconditioned stimulus or neutral conditioned stimuli. Contrary to a number of previous studies that found increased emotional reactions to trauma reminders in PTSD patients
(6,
7), our study showed no significant group differences in self-reported, peripheral, and brain responses to the trauma-specific unconditioned stimulus. The lack of expected group differences in the responses to the trauma-specific unconditioned stimulus might be based on a particularly aversive evaluation of the unconditioned stimulus in healthy comparison subjects rather than a dampened response in PTSD patients as evident from the high arousal and unpleasantness ratings and the clear conditioned-unconditioned stimulus differentiation prior to conditioning in all three groups. This exaggerated response in healthy comparison subjects might have been because of the fact that the traumatic event was an important and tragic incident in Germany, particularly in the region where the experiment was carried out. In addition, the unconditioned stimulus was chosen to be highly aversive in a large cohort of healthy comparison subjects. The elevated response in healthy comparison subjects assured that both the traumatized subjects and the nontraumatized comparison subjects showed a high unconditioned response to the trauma picture, thus setting the prerequisite for a stringent evaluation of conditioning to the trauma stimulus.
The altered conditionability of the traumatized subjects and the failure to extinguish among PTSD patients is thus not the result of different physiological or self-reported emotional reactions to the unconditioned stimulus prior to the acquisition phase, but rather a consequence of differences in the associative learning process itself.
The findings of this study must be interpreted in light of some limitations. One of the main strengths of the study, the inclusion of various response levels, also bears the problem of multiple testing and thus alpha-error inflation. Although within-group paired-comparisons and between-group post hoc tests were Bonferroni-corrected for multiple comparisons, we did not correct for the number of dependent variables. However, we feel that the advantage of multiple indicators of the conditioned response as well as the modest overall cohort size justify this less rigorous approach.
The second major limitation of the study refers to the repeated presentation of an identical visual unconditioned stimulus, which was chosen to maximize standardization. However, self-reports related to the unconditioned stimulus did not habituate significantly over time.
To our knowledge, these findings are the first to show differential second-order conditioning to aversive trauma-specific cues in PTSD patients and traumatized persons without PTSD relative to healthy comparison subjects. PTSD patients displayed higher conditioned responses that did not extinguish, whereas trauma-exposed persons without PTSD showed similar conditionability but a quick extinction of the conditioned response. In contrast, trauma-relevant aversive cues did not lead to conditioned responses in healthy comparison subjects. These data support recent animal models
(38) and etiological theories of PTSD that favor a Pavlovian conditioning account
(2,
39) and lend special credence to the assumption of deficient extinction
(40) .
Our results extend previous findings, since they suggest that PTSD may be maintained by second-order conditioning processes where trauma-relevant cues come to serve as unconditioned stimuli and by confirming a special role of a lack of extinction. This finding underlines the importance of therapies that focus on the extinction of learned responses, such as behavioral treatments, that may be enhanced by the use of pharmacological substances that foster extinction of a learned aversive response
(41) .
Although less intense second-order conditioning was also present in trauma-exposed subjects without PTSD, these subjects did not show a failure of extinction similar to the PTSD group, suggesting that the failure to extinguish the conditioned response may be the important variable for the maintenance of PTSD, whereas first- and second-order conditioning might be inherent to the traumatic event itself. Prospective studies in high-risk populations are needed to clarify whether the altered conditionability of PTSD patients is a predisposing factor for the development of the disorder, a maintaining factor, or a result of symptom chronicity.