Genetic as well as environmental factors influence susceptibility to CNS infections, which are associated with schizophrenia as suggested by some evidence.
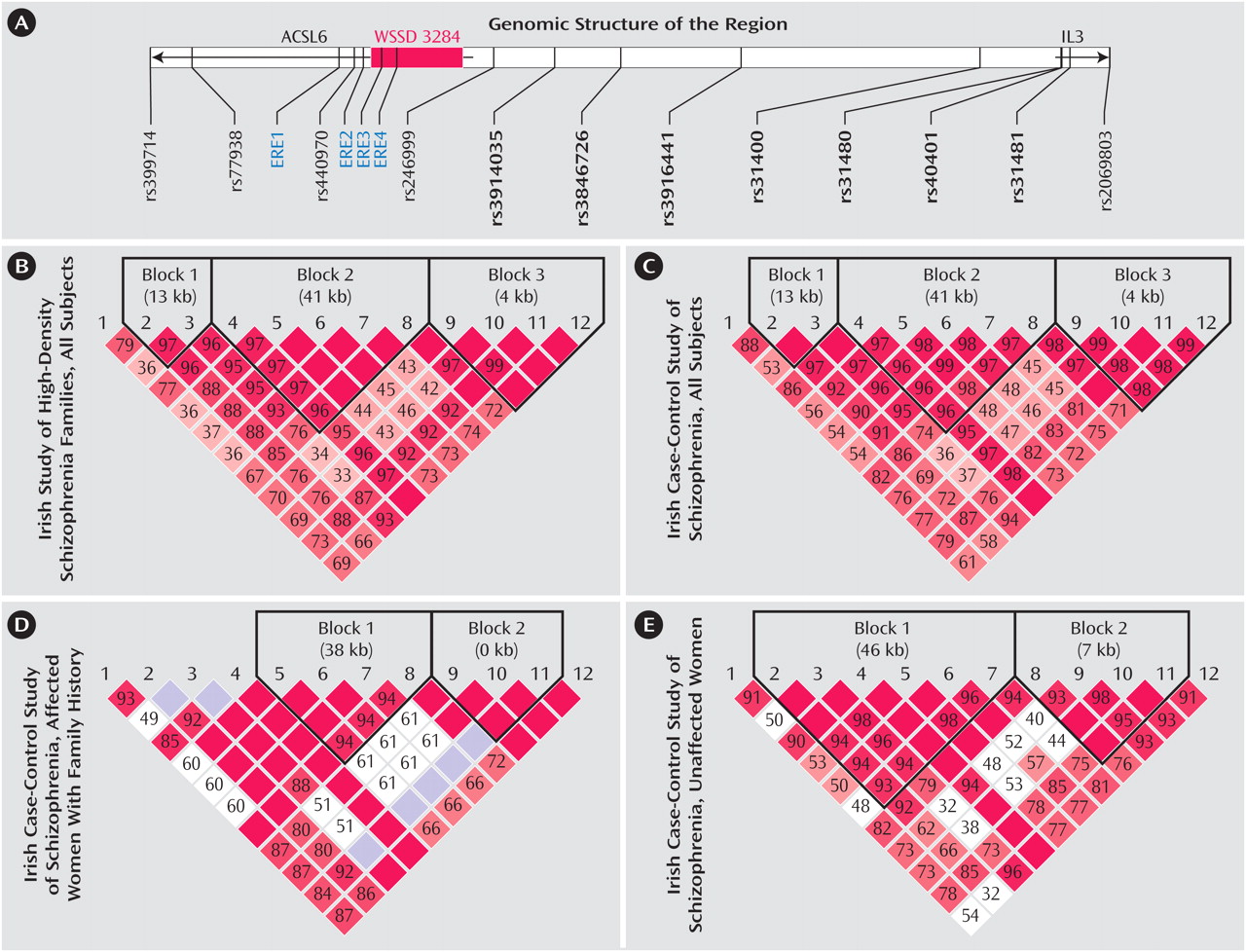
Interleukin 3 (IL3) is a cytokine that has multiple functional roles, including an involvement in neural development, stimulation of cell growth, suppression of apoptosis, and enhancement of hematopoiesis. IL3 is a major component of the immune system, a system long suspected of playing an etiological role in schizophrenia. Moreover, abnormal activities of several interleukins, including IL3, have been reported in schizophrenia. We investigated whether genetic variants in the IL3 gene might be associated with schizophrenia by typing single nucleotide polymorphisms (SNPs) in and around this gene. SNPs are common variations in DNA sequence at a specific position where a particular base—for example, an adenine (or A)—is present in some individuals, but a different base—guanine (or G)—is present in others. We indeed found evidence that several SNPs in and around the IL3 gene were associated with schizophrenia in two different samples: the Irish Study of High-Density Schizophrenia Families and Irish Case-Control Study of Schizophrenia. We studied 12 SNPs in and around the following two genes: ACSL6 and IL3 (see panel A for the genomic structure of the region). Some specific variants of these SNPs (called “alleles”—that is, an A, T, C, or G) and their combinations (called “haplotypes”—small chunks of DNA that have traveled together over evolutionary time) were overrepresented in the affected individuals relative to unaffected comparison subjects. Furthermore, these associations were sex-specific (limited to female subjects) and restricted to those individuals with a positive family history (FH) of illness. In the analysis of linkage disequilibrium (LD)—a measurement of non-random association of alleles at two or more genetic loci—the two samples showed similar patterns (panels B and C), suggesting that the two samples have similar underlying allele and haplotype structures for these SNPs. This underlying structure is largely a result of evolutionary processes, particularly how prior chromosomal crossovers that occur during meiosis in eggs and sperm are distributed. In the subgroup of patients showing associations for the Irish Case-Control Study of Schizophrenia study, women with a family history of illness showed some differences in the LD pattern (panels D and E). In the figure panels B-E, the intensity of the red color reflects the magnitude of the statistically significant LD between marker pairs (from 0 to 100; red cells with the maximal LD of 100 have no number). In the interval between rs246999 and rs31480 (panel A), there are several DNA sequence motifs (promoter and enhancer) capable of binding to known transcription factors (such as AP-1, SP1, and GATA) that regulate the expression of the IL3 gene. There are four estrogen response elements (ERE) in this region, and two of them are located in a segmental duplication (WSSD 3248). These EREs and their potential change in copy number may be responsible for the female-specific associations. These data suggest that genetic variations in this region may cause change of IL3 expression, which in turn could alter the risk for schizophrenia. Changes in IL3 expression could influence risk for schizophrenia by several mechanisms. IL3-modulated changes in the rate of apoptosis during development could alter neuronal populations in the adult brain. Alternatively, IL3, through its regulation of activated microglia, could influence inflammatory responses in the developing or adult brain, thereby altering the vulnerability of an individual to an infectious process.