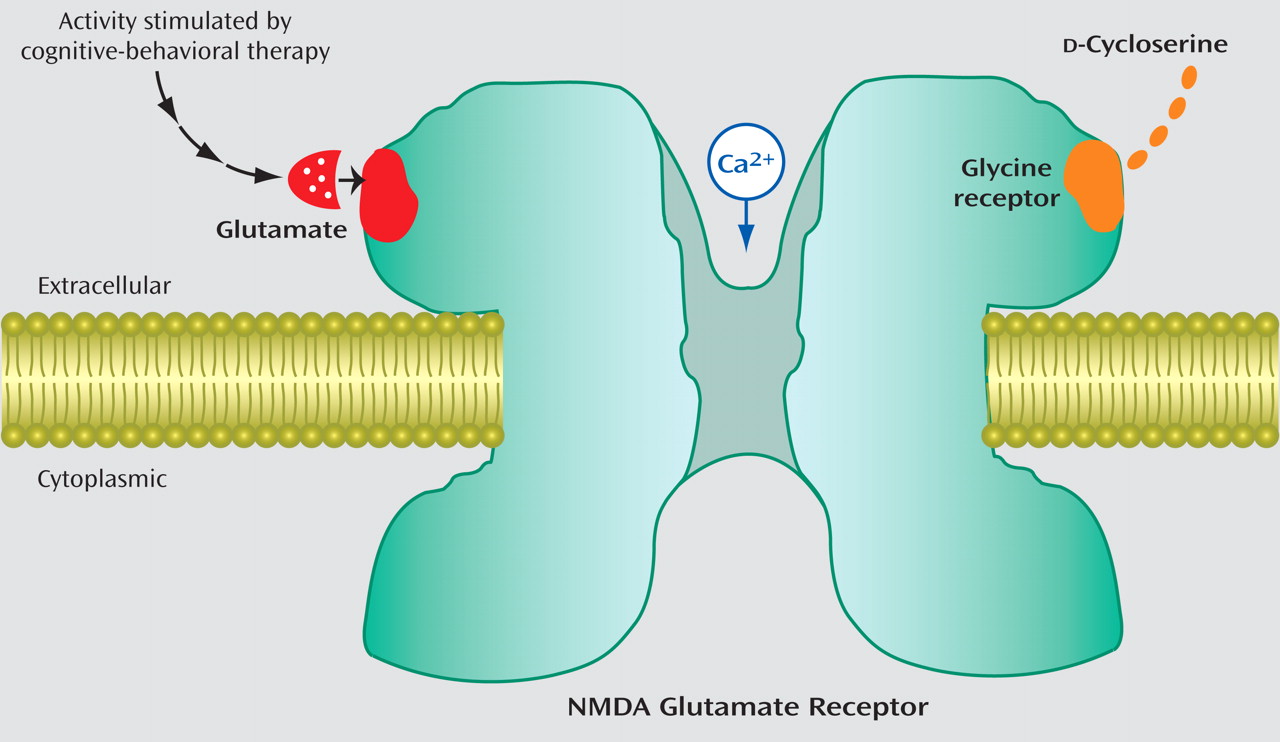
The study by Wilhelm et al. in this issue in which
d -cycloserine is used to facilitate exposure therapy for obsessive-compulsive disorder (OCD) confirms an earlier report showing the efficacy of this treatment strategy for OCD and represents yet another example of the paradigm shift that is taking place in psychiatry that combines cognitive enhancers with cognitive-behaviorial therapy (CBT). In this study, the use of a
d -cycloserine cognitive enhancer in combination with cognitive-behavioral therapy facilitated the treatment of OCD, finding differences between
d -cycloserine and placebo on the Yale-Brown Obsessive Compulsive Scale at mid-treatment and depression at posttreatment. It is a paradigm shift because it is a direct attempt to stimulate chemically the
N -methyl-
d -aspartate (NMDA)-glutamate synapses that are thought to be the critical nerve cell mechanisms that support short-term learning and memory at the same moment that CBT is being used to help the patient learn new behaviors (
Figure 1 ).
d -Cycloserine is a unique partial agonist for this purpose because it is thought to work cooperatively with the glutamate that is presumably being released through synaptic activity associated with the patient’s participation in CBT.
Adding “traditional” medications to CBT has shown no advantage over CBT alone for the three anxiety disorders that have been treated using CBT plus
d -cycloserine. Three studies evaluated combined “standard” pharmacotherapy (e.g., alprazolam, buspirone, imipramine) and CBT approaches for panic disorder
(1 –
3) . None showed an advantage of medication over CBT or of adding medication to CBT. In the five controlled trials combining traditional medications (i.e., fluvoxamine, sertraline, or clomipramine) with CBT for OCD, there was no clear advantage for combined treatment over CBT alone
(4 –
8) . There was no evidence for greater benefit of combined treatment over CBT alone for social phobia in the three randomized, controlled trials that combined treatments using sertraline
(9) or fluoxetine
(10,
11) . In posttraumatic stress disorder, an advantage was found for adding exposure therapy to sertraline only for patients who had an incomplete response to the medication alone
(12) .
Many of the CBT treatments include some form of exposure therapy, and exposure therapy is recommended for most, if not all, of the anxiety disorders. Exposure therapy involves confrontation with feared stimuli in a therapeutic manner and has its basis in the animal literature in extinction training. By presenting the feared stimuli repeatedly with no aversive consequences, the fear decreases until ideally, the stimuli no longer engender fear.
Advances in animal research elucidating neurotransmitters involved in fear extinction have led to the exploration of combination treatment strategies for anxiety disorders focusing on the enhancement of extinction learning that occurs in CBT. In this model, pharmacotherapy is aimed at improving the learning that takes place during CBT (specifically exposure-based therapy) and not at treating the symptoms of anxiety
(13) . The glutamatergic NMDA receptor has been found to be critically involved in learning and memory (e.g.,
14,
15), and this learning may be augmented by the NMDA partial agonist
d -cycloserine.
d -Cycloserine has been shown in animal studies to facilitate the extinction of learned fear and to produce generalized extinction
(13) .
d -Cycloserine has been approved by the Food and Drug Administration (FDA) for the treatment of tuberculosis for over 20 years, and the side effect profile indicates no adverse effects of single-pill administrations.
As reviewed in the article by Wilhelm et al., the first clinical test of a treatment for a specific phobia in humans that combined
d -cycloserine with exposure therapy was performed by Ressler et al.
(16) . Participants underwent two therapy sessions (suboptimal exposure) using virtual reality exposure therapy and were instructed to take a single pill 2–4 hours before the therapy session, for a total of two pills during the course of the study. Participants who received
d -cycloserine in conjunction with exposure therapy had significantly less fear within the virtual environment, reported significantly more improvement in their overall acrophobic symptoms at the 3-month follow-up, and displayed significantly more improvements in psychophysiological measures of anxiety than the placebo group.
Two studies have found advantages of
d -cycloserine over placebo when added to exposure therapy for social phobia
(17,
18) . Both studies used 50 mg of
d -cycloserine administered 1 hour before therapy sessions, with four exposure therapy sessions. The study with panic disorder patients used 50 mg of
d -cycloserine 1 hour before three sessions of exposure therapy and found advantages of
d -cycloserine over placebo (unpublished study by Tolin et al.).
The results of the now three trials testing
d -cycloserine with OCD are mixed, but as Wilhelm et al. (this issue) discuss, it is likely due to methodological differences. The Wilhelm et al. study used 100 mg dosed 1 hour before 10 exposure therapy sessions and found benefits for OCD on the Yale-Brown Obsessive Compulsive Scale (a standardized measure of OCD) at mid-treatment and for depression at posttreatment. The other study to find advantages of
d -cycloserine for OCD used 125 mg of
d -cycloserine 2 hours before 10 exposure sessions and found differences at mid-treatment on subjective units of discomfort on a self-report anxiety scale, but the Yale-Brown Obsessive Compulsive Scale was only administered at posttreatment, at which point no group differences were found
(19) . In the negative study of
d -cycloserine for OCD
(20), 250 mg of
d -cycloserine was given 4 hours before 12 sessions of 90-minute therapy. These differences in methodology and outcome are very illuminating and suggest that important variables include 1) the timing of dosing—dosing too early may lead to the peak drug effect not being coincident with the emotional learning processes that take place during and immediately after psychotherapy sessions, 2) the dose of drug—too high a dose may activate the antagonist properties of this NMDA partial agonist, and 3) a floor effect of all the subjects improving from a full course of therapy so that no effect of drug by therapy effect can be observed.
It is important in translational research that we not change parameters that have been shown in the animal studies to be determinants of outcome. In the d -cycloserine literature thus far, it seems to be that less d -cycloserine is better than more d -cycloserine (dose and frequency of dosing), and the timing of the dosing is critical. Lower doses seem to be more effective than higher doses. Too frequent dosing (daily) seems to be ineffective. Possibly too many doses, even well spaced, may also nullify the effects. And finally, it may be more important to have the medication on board immediately following the exposure/extinction training during the consolidation phase than during the extinction training phase, so it is important to administer the medication close to the onset of the session so that it will still be active following the session. These conclusions have been borne out in a recent meta-analysis that confirmed that d -cycloserine is effective in animals and humans (unpublished study by Norberg et al.).
There are several different strategies for combining pharmacotherapy and psychotherapy and challenges and advantages to each. For the antidepressant medications, if they are added at the start of CBT, there is the problem of time to response, generally about 4 weeks, for the medication, and the extant literature does not suggest any advantage to combining these medications with CBT for anxiety disorders. It may make more sense to combine these approaches sequentially, starting first with the antidepressant and having it on board at least 4 weeks before commencing the psychotherapy and adding in CBT only for those with a partial response to the medication. For anxiolytic medications other than the antidepressants, generally the onset of action is much quicker, and therefore, they can be combined with psychotherapy from the onset. However, there is some evidence that they may impede CBT, especially exposure therapy. For the more novel medication approaches such as d -cycloserine, the drug is not expected to afford any benefits in and of itself; it is only in combination with the exposure therapy, so they should be on board at the same time.
In contrast to the more traditional medications (e.g., antidepressants and anxiolytics) combined with CBT for anxiety, these novel medication approaches do seem to afford some advantages when combined with CBT. The new approaches aim to combine a cognitive enhancer medication that will specifically enhance the efficacy of the emotional learning process that takes place in psychotherapy and hopefully make these new emotional memories more robust, stable, and long-lasting. What is particularly promising about this approach is that it was developed rationally, based on the neuroscience of fear and anxiety and extinction, rather than spuriously. It was serendipitous that this particular NMDA partial agonist shown to be effective in facilitating the extinction of fear in animal research had been FDA-approved for humans, speeding up the application to humans. We have seen a surge in rational pharmacotherapy based on the neuroscience, some of which has not been borne out (e.g., substance P antagonism and major depressive disorder), and other approaches that are very hopeful (e.g., corticotropin-releasing factor receptor antagonists for treating anxiety). In this age of “bench to bedside” translational research, we are sure to see more translational approaches to the treatment of psychiatric disorders.