In addition to actions on the hypothalamic-pituitary-adrenal (HPA) axis, corticotropin-releasing hormone (CRH) acts as a neurotransmitter or neuromodulator to coordinate stress-induced neural responses in the brain
(1) . High-affinity CRH 1 and 2 receptors (CRH
1 and CRH
2 ) and CRH mRNA are heterogeneously distributed throughout the brain
(2) . Many of the behavioral effects observed in animals after central CRH administration closely resemble the symptoms of depression in humans
(3), including behavioral despair, disrupted sleep patterns, decreased sexual behavior, and decreased food consumption. Certain stressors also elicit the responses cited above, and many of these effects of stress are attenuated by central administration of alpha helical CRH-(9-41), a specific CRH receptor antagonist
(4) .
Clinical findings further support the hypothesis that CRH may be hypersecreted in psychopathologic states. For example, CSF levels of CRH are elevated in depressed patients
(5) and return toward normal 24 hours after a course of treatment with ECT
(6) . In addition, a 40% incidence of dexamethasone nonsuppression has been described in depressed patients
(7), and adrenal gland hypertrophy has been observed in severely depressed individuals
(8) . One postmortem study of CRH
1 receptor densities in the brains of suicide victims found diminished CRH
1 receptor binding capacity
(9), which supports the hypothesis that brain CRH systems may be hyperactive in individuals with severe melancholia.
One clinical trial of a CRH
1 receptor antagonist (R121919) in the treatment of major depression has been published
(10) . Data from this trial indicated significant reductions in scores on the 21-item Hamilton Depression Rating Scale (HAM-D) over the 30-day treatment period. However, this pilot study did not include design components such as blinding, randomized treatment allocation, and placebo control, thereby limiting the conclusions that may be drawn regarding an antidepressant effect of R121919.
CP-316,311 is a selective CRH 1 receptor antagonist that readily enters the CNS after oral administration and binds with nanomolar affinity to human CRH 1 receptors (50% maximal inhibitory concentration [IC 50 ]=0.4–1.7 ng/ml), with a projected clinically efficacious serum exposure in the range of 267–1133 ng/ml (assuming that free brain concentration is equal to free plasma concentration). Functional antagonism at the CRH 1 receptor in animal models is supported by blockade of responses elicited by exogenous CRH and stressful stimuli that activate endogenous CRH systems; for example, in the rat fear-potentiated startle model, the effective dose for 50% of subjects (ED 50 =3.2 mg/kg) was associated with a projected clinically efficacious concentration (EC 50 ) of 100 ng/ml in humans.
Phase 1 pharmacokinetic data projected steady-state mean serum trough concentrations of approximately 978 ng/ml with the dosage of CP-316,311 used in this study (400 mg twice daily), thus exceeding in vivo projections and approximating the upper end of in vitro (human CRH 1 receptor affinity) projections for required clinically efficacious serum exposures. A CSF study in healthy subjects confirmed that CSF and plasma concentrations of CP-316,311 were similar to the free fraction in human plasma determined ex vivo before this trial was conducted.
Method
Study Design
The study included an initial 1-week single-blind placebo treatment phase and a 6-week fixed-dose, double-blind, double-dummy, parallel-group treatment phase. The primary comparison in the study was between placebo and CP-316,311 at a dosage of 400 mg twice daily. Sertraline at a dosage of 100 mg/day was included as a positive control. A group sequential design was used to allow the study to be stopped early for futility or utility at a planned interim analysis. The study was conducted at 18 centers in the United States, Serbia and Montenegro, and the Russian Federation.
Participants
The study included male and female outpatients at least 18 years of age with a diagnosis of recurrent major depressive disorder (DSM-IV 296.3x), a score ≥28 on the Montgomery-Åsberg Depression Rating Scale (MADRS; 11), a score ≥4 on the Clinical Global Impression (CGI) severity item at the screening and baseline visits, and a score ≥12 on the Hamilton Anxiety Rating Scale (HAM-A) at baseline. Females of childbearing potential were excluded because of preclinical findings indicating a risk of embryo-fetal development toxicity with CP-316,311. Participants were randomly assigned in a 1:1:1 ratio to receive 400 mg of CP-316,311 twice daily, placebo, or 100 mg of sertraline daily (50 mg/day for the first 3 days and 100 mg/day thereafter).
Efficacy Evaluations
The primary efficacy measure was change in total score from baseline to last observation on the 17-item HAM-D. Secondary efficacy evaluations included rates of response and remission according to the HAM-D (response=50% reduction in total score from baseline; remission=score ≤7), the MADRS (response=50% reduction in total score from baseline; remission=total score ≤9), and the CGI improvement item (response: score of 1 or 2) as well as change from baseline to last observation on the MADRS, the HAM-A, and the CGI severity item.
Safety Evaluations
Safety evaluations included measurement of vital signs and weight, physical and neurological examinations, 12-lead ECG, monitoring of adverse events, and safety laboratory tests. Morning urinary cortisol was assayed as a biomarker of HPA function.
Pharmacokinetic Evaluations
Trough pharmacokinetic samples were collected prior to first dose and thereafter before dosing at weeks 1, 2, 4, and 6.
Statistical Analyses
A group sequential design was used to permit early stopping of the trial if efficacy-based futility or utility was established at a planned interim analysis, when approximately 50% (99 subjects of 198 projected) either completed or discontinued the study. The group sequential method in the software program East, version 4 (Cytel Inc., Cambridge, Mass.) was used to perform the interim analysis. The spending function approach (for both alpha and beta spending) by Lan and DeMets
(12) with O’Brien-Fleming-type boundaries was used. The cumulative one-sided alpha for the study was maintained at 10%. The overall power for detecting a 3-point improvement of CP-316, 311 over placebo on the HAM-D (assuming a common SD of 7.5) was approximately 83%.
The primary analysis (on the interim and final data sets) was performed using data from all participants who received at least one dose of trial medication and for whom HAM-D total score was available for the baseline and week 1 visits. The HAM-D total score change from baseline at week 6 was analyzed using a linear model (last observation carried forward). The model included treatment, center, and HAM-D baseline score as independent variables. The primary treatment comparison was between CP-316,311 and placebo. A secondary comparison between sertraline and placebo was performed to evaluate the assay sensitivity of the trial.
Secondary efficacy analyses were performed only on the final study data set. These included change from baseline on the MADRS, the CGI severity score, and the HAM-A; response and remission analyses on the HAM-D and the MADRS; and response analysis of the CGI improvement item.
Results
Interim Analysis Results
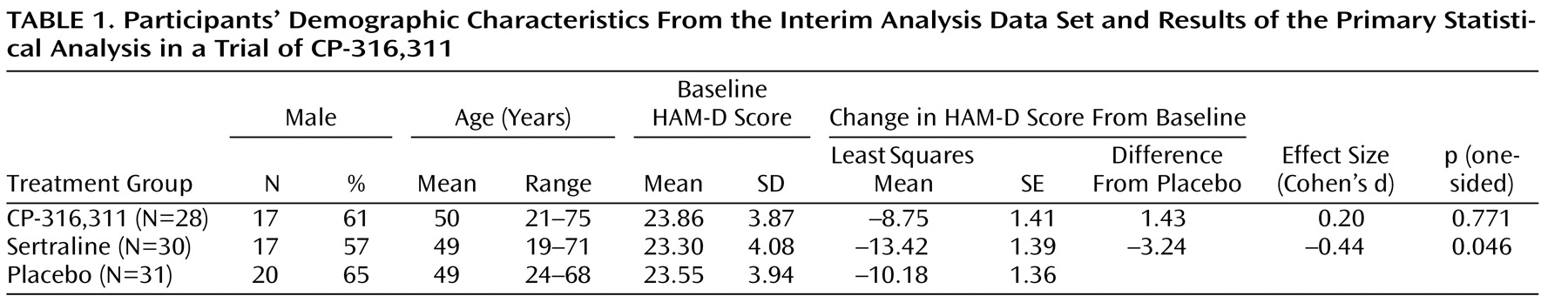
The interim analysis was performed after 90 participants were accrued. Of these 90 participants, 89 were evaluable for efficacy. There were no between-group differences in demographic characteristics in the interim analysis (
Table 1 ). The primary statistical analysis of the HAM-D (analysis of covariance with baseline as a covariate) is summarized in
Table 1 .
At the interim analysis, the one-sided p value comparing the CP-316,311 and placebo groups was 0.77. This crossed the p value boundary (0.57) for rejecting the alternative hypothesis that CP-316,311 was superior to placebo, and the trial was stopped for futility.
Final Data Set Results
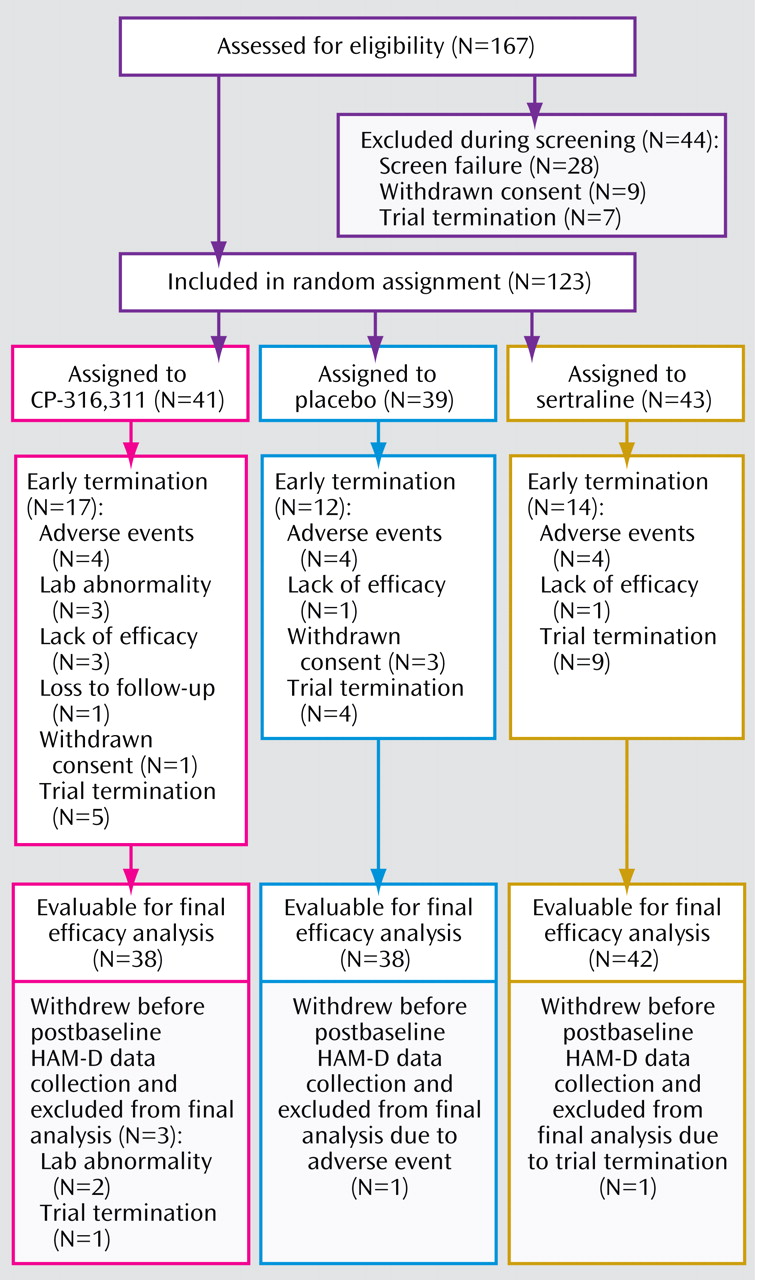
Since the final data set included participants who were discontinued once futility was declared, results are included here for descriptive purposes only. Participant disposition is summarized in
Figure 1 .
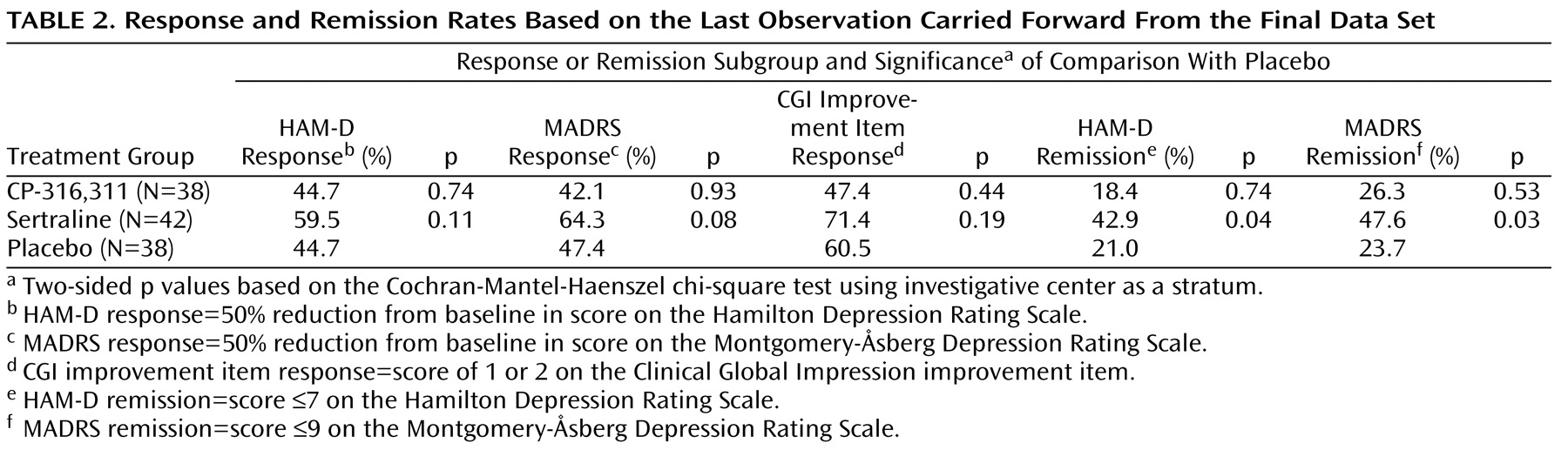
There were no between-group differences in demographic characteristics in the final data set. Results on the primary endpoint were consistent with those of the interim analysis. The CP-316,311 least squares mean difference from placebo was 1.06 (p=0.53), and the sertraline least squares mean difference from placebo was –2.47 (p=0.14). The least squares mean for the placebo group was –10.35. Secondary last-observation-carried-forward analyses of scores on the HAM-A, the MADRS, and the CGI severity item confirmed lack of efficacy for CP-316,311 on these endpoints, and last-observation-carried-forward analyses of response and remission rates using the HAM-D, the MADRS, and the CGI improvement item further supported the interim analysis conclusions of futility (
Table 2 ) and hence of a negative trial for CP-316,311.
Pharmacokinetic Results
A total of 160 pharmacokinetic samples were collected from 41 participants in the CP-316,311 group; 73% of these samples were collected 10–14 hours after dosing. Mean trough concentrations from these samples ranged from 1204 ng/ml (week 1) to 1827 ng/ml (week 6), which were above the projected human efficacious serum concentrations relative to in vivo (∼100 ng/ml) and in vitro (267–1133 ng/ml) projections.
Safety Results
Adverse events reported in more than one participant in the CP-316,311 group included depression (7.3%), anxiety (4.9%), insomnia (4.9%), and nausea (4.9%). Two participants in the CP-316,311 group withdrew because of treatment-related laboratory abnormalities: one due to suspected hypothyroidism and the other due to increased AST/ALT at baseline (before randomization). No clinically significant differences in other safety parameters (including liver function tests) were noted between treatment groups.
Participants in the CP-316,311 group had a mean decrease in urinary cortisol levels (–30.4 nmol/liter) compared with the placebo group (0.7 nmol/liter), which approached statistical significance (p=0.09, two-sided). No changes were noted in the sertraline treatment group.
Conclusions
In this trial we obtained a negative result for CP-316,311 in the treatment of major depressive disorder. The trial validity was supported by the secondary comparison of sertraline and placebo at the interim analysis, which was consistent with published reports for sertraline in major depression
(13) . Trough serum concentrations of CP-316,311 at week 1 and week 6 indicated that adequate serum exposures were achieved relative to in vitro and in vivo estimates of required exposure to test the protocol-specified hypothesis. Furthermore, effects of CP-316,311 on urinary cortisol provided supportive evidence of central CRH
1 receptor antagonism.
Study limitations include a lack of generalizability of these results to female subjects of childbearing potential, since they were excluded from the trial.