Cognitive reserve has been proposed as an important etiologic factor in the development and severity of neuropsychiatric disorders
(1) . The construct of cognitive reserve refers to individual differences in brain structure (e.g., density of neuronal synapses) and function (e.g., processing efficiency) thought to buffer the effects of neuropathology. Evidence has emerged from the rapidly evolving field of cognitive epidemiology
(2) showing that IQ, a marker of cognitive reserve, is inversely related to risk of total psychiatric illness
(3) . However, with the exception of research on schizophrenia
(4), surprisingly few studies have examined the relation between IQ early in life and the risk of other specific adult psychiatric disorders. Among those studies that have examined other disorders, the results have been inconclusive. We report a longitudinal study of the 20-year predictive association between childhood IQ and adult mental disorders among members of the Dunedin, New Zealand, birth cohort.
In the earlier studies, low IQ at military entry increased the risk of hospitalization for depression in male Swedish conscripts
(5) but not male Danish conscripts
(6) . Studies of bipolar disorder have largely shown no association with premorbid IQ
(5,
7,
8), but one study of U.S. Army recruits found elevated IQ scores in bipolar patients
(9) . Low IQ has been associated with increased risk of substance use disorders in some study groups
(6,
10) but not in others
(11 –
13) . In one cohort, the association between low childhood IQ and increased risk of any anxiety disorder was attenuated to nonsignificance once other covariates were included
(11) . An inverse association between premorbid IQ and posttraumatic stress disorder (PTSD) has been consistently documented in military
(14) and civilian samples, including our Dunedin birth cohort
(15) . However, with the exception of one report showing that lower IQ increased the risk of generalized anxiety disorder
(16), the association between premorbid IQ and other specific anxiety diagnoses has not been studied.
Understanding of the potential role of cognitive reserve in the etiology of neuropsychiatric disorders is constrained by five methodological limitations of extant research. First, many studies have relied on IQ at military induction as a marker of cognitive reserve
(5,
6) . Such studies cannot rule out the possibility that low IQ at induction is the consequence of prior juvenile disorder or an early prodromal disease process, rather than antecedent to disorder. Prospective studies using IQ measured in childhood provide a more rigorous test of whether lower premorbid IQ increases the risk of subsequent disorder. Second, most prior studies have considered either a single or limited number of disorders, making it difficult to determine whether low IQ is a general risk factor for all psychiatric disorders or a specific risk factor for select disorders with a neurodevelopmental etiology (e.g., schizophrenia). Third, studies including a range of diagnoses have tended to rely on hospitalization records for their psychiatric outcome data
(5,
6), which may introduce ascertainment biases. Only individuals with the most severe forms of nonpsychotic disorders are hospitalized, and thus hospitalized patients do not represent nonpsychotic disorders in the population. If low IQ increases the likelihood of hospitalization, the association between premorbid IQ and psychiatric disorders would be inflated. Fourth, several of the most rigorous studies have used male samples
(5,
6,
14) . Studies in cohorts representing both sexes are needed. Fifth, prior studies have predicted the presence of either lifetime disorder or disorder at the time of assessment. However, the cognitive reserve model proposes that low IQ should be associated with the severity of psychopathology, not merely the presence versus absence of a diagnosis. Indicators of disorder severity, such as comorbidity and persistence, should be studied.
In order to test the cognitive reserve model for psychiatric disorders, we examined whether childhood IQ predicted psychiatric disorders at the most recent follow-up of the Dunedin birth cohort, at age 32. Focusing on age 32 allowed greater confidence in the validity of certain diagnoses, such as substance dependence and schizophrenia, which can be difficult to make at earlier ages in cohort studies. To allow comparison with prior reports of the association between IQ and adult disorder at one time point, we report this association between childhood IQ and past-year disorder assessed at age 32. However, we also capitalized on the longitudinal design of our study and report the relationship between childhood IQ and the persistence of disorder across repeated assessments carried out from age 18 to 32. This study overcomes several methodological limitations by using a birth cohort of both men and women who had prospective data on childhood IQ and potential confounders and who were diagnosed with specific mental disorders by means of structured clinical interview rather than hospital records. In addition, it is one of the first studies to examine the relation between IQ and indicators of disorder severity.
Method
Participants
The participants were members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of the health and behavior of a complete cohort of children born during a 1-year period in 1972–1973 in Dunedin, New Zealand. The assessment at age 3 included 1,037 individuals (52.0% male, 91.0% of eligible births), who formed the base sample for the longitudinal study. The cohort members represent the full range of socioeconomic status in the general population of New Zealand’s South Island, and they are primarily white. The cohort has been assessed at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, and most recently, at age 32, when 972 participants (96.0% of living cohort members) were assessed. The research procedure involved bringing each study member (including emigrants living overseas) to the research unit for an 8-hour day of individual interviews and tests. Each research topic was presented as a standardized module by a different trained examiner in counterbalanced order throughout the day. After description of the study, informed consent was obtained.
This study protocol was approved by the institutional review boards of the Institute of Psychiatry, Duke University, and Dunedin School of Medicine. The data analysis was approved by the human subjects committee at the Harvard School of Public Health. Study members gave informed consent before participating.
Measures
IQ was assessed at ages 7, 9, and 11 years by means of the WISC-R
(17) . The IQs determined at the three ages were averaged and standardized.
Mental disorders were assessed in private, standardized interviews by using the Diagnostic Interview Schedule (DIS)
(18), with a reporting period of 12 months at each age, as described previously
(19) . At ages 26 and 32, diagnoses followed the DSM-IV criteria. At ages 18 and 21, diagnoses followed the then-current DSM-III-R criteria. Psychiatric disorders assessed at ages 18 through 32 included schizophrenia spectrum disorder, manic episode, major depression, any anxiety disorder, and cannabis, alcohol, and other drug dependence; the last three were assessed at ages 26 and 32 only. The specific anxiety disorders included were generalized anxiety disorder, social phobia, PTSD (ages 26 and 32 only), agoraphobia, panic disorder, specific phobia, and obsessive-compulsive disorder (OCD). Interviews were conducted by experienced clinicians who had tertiary degrees in social work, medicine, or clinical psychology (not lay interviewers) and who were blind to the study members’ IQ and psychiatric history. The 12-month prevalence rates for the Dunedin cohort
(19) are comparable to 12-month rates from the National Comorbidity Survey
(20) .
The primary outcomes for this analysis were 12-month diagnoses at age 32. Exceptions to the 12-month time frame were manic episode and schizophrenia spectrum disorder, which have been diagnosed in the larger study as lifetime disorders, as will be described. Manic illness was diagnosed if the participant met the formal DSM-IV criteria through the study’s DIS interviews (N=8, 0.8%). All participants diagnosed with mania in the study by age 32 also had been diagnosed outside the study and had received prescriptions for lithium or sodium valproate. Methods to enhance our research diagnosis of schizophrenia have been described previously
(21) . By age 32, 1.1% of the sample (N=11) met all of the criteria for schizophrenia and had been hospitalized and/or treated with antipsychotic medications. A further 2.5% (N=24) likewise had met all of the criteria for schizophrenia but had not yet been registered in the New Zealand health service as a schizophrenia patient. This Dunedin cohort prevalence of 3.6% (N=35) may seem high, but it is close to the 3.5% recently reported as the lifetime prevalence of psychosis in the general population
(22) . We conservatively use the term “schizophrenia spectrum” because ours is a research diagnosis and not a clinical diagnosis.
The analyses reported here of persistent disorder across repeated prospective assessments reveal a high cumulative lifetime prevalence of certain disorders in the Dunedin cohort, as compared to lifetime prevalence estimates for the same age group in retrospective surveys such as the National Comorbidity Survey
(20) . However, although prospective and retrospective studies show markedly different cumulative prevalences, they show similar past-year prevalences. For example, major depression has a 14% past-year prevalence across ages 15 to 32 in the Dunedin cohort, which is similar to the 12% past-year prevalence for the 15–34 age group in the National Comorbidity Survey
(20) . The Dunedin cumulative prevalence is a summation of all the waves of prospectively diagnosed episodes. Evidence suggests that assessing disorders retrospectively underestimates lifetime prevalences because of recall bias
(23) .
Confounders
Variables that could be considered prior causes common to both low IQ and adult mental disorders were included as confounders in our models. These were childhood socioeconomic status, number of perinatal insults, low birth weight, and childhood maltreatment. Childhood socioeconomic status was measured with a scale that places parental occupation into one of six categories based on the educational level and income associated with that occupation in the New Zealand census
(24) . The correlation between childhood socioeconomic status and IQ was significant (r=0.41, N=986, p<0.001). The number of perinatal insults included a count of 12 prenatal and 12 neonatal problems recorded by clinicians during the mother’s pregnancy
(25) . Participants were coded as having none, one, or two or more perinatal insults. The correlation between perinatal insults and childhood IQ was also significant (r=–0.07, N=991, p<0.05). Low birth weight was defined as a birth weight below 2.50 kg; this too was significantly correlated with childhood IQ (r=–0.06, N=991, p<0.05). Childhood maltreatment has been described previously
(26) . Briefly, the measure includes assessment of 1) maternal rejection at age 3 years, 2) harsh discipline at ages 7 and 9 years, 3) two or more changes in the child’s primary caregiver, and 4) physical and sexual abuse reported by study members once they reached adulthood. Our cumulative exposure index for each child is a count of the number of maltreatment indicators during the first decade of life: 64.2% of the children experienced no maltreatment, 26.6% experienced one indicator of maltreatment, and 9.2% experienced two or more indicators of maltreatment. The correlation between childhood maltreatment and IQ was significant (r=–0.13, N=990, p<0.001).
Data Analysis
First, using logistic regression, we tested whether lower childhood IQ predicted an increased risk of a diagnosis at age 32. The comparison group comprised healthy participants, i.e., those not diagnosed with any psychiatric disorder at age 32 (N=583). Because there are sex differences in the prevalences of specific psychiatric disorders, we adjusted the model for each diagnosis for sex. We then ran the model after adjusting for sex and the remaining confounders (socioeconomic status, number of perinatal insults, low birth weight, childhood maltreatment). The strength of the associations between childhood IQ and adult disorders is presented by odds ratios with 95% confidence intervals (CIs).
Second, we investigated the relationship between childhood IQ and severity of psychiatric illness, operationalized by comorbidity and persistence. Comorbidity was defined as the number of diagnostic families for which a participant met the criteria at age 32. The four diagnostic families were as follows: any psychotic disorder, any mood disorder, any anxiety disorder, and any substance dependence. Using logistic regression, we tested whether childhood IQ predicted having psychiatric disorders from two or more diagnostic families at age 32. Persistence was defined as having met the criteria for a disorder at two or more assessments across ages 18, 21, 26, and 32. Persistence was defined for the following disorders: major depressive episode, any anxiety disorder, generalized anxiety disorder, social phobia, agoraphobia, panic disorder, simple phobia, OCD, cannabis dependence, and alcohol dependence. Other diagnostic groups were either too small to be subdivided for the persistence analysis or were not assessed at all four waves. In separate logistic regressions, we tested whether childhood IQ predicted having two or more episodes of a disorder among participants who were diagnosed with the disorder at least once. The strength of the associations between childhood IQ and both comorbidity and persistence is represented by odds ratios with 95% CIs. The effect size for childhood IQ for all analyses is presented as Cohen’s d
(27) .
Results
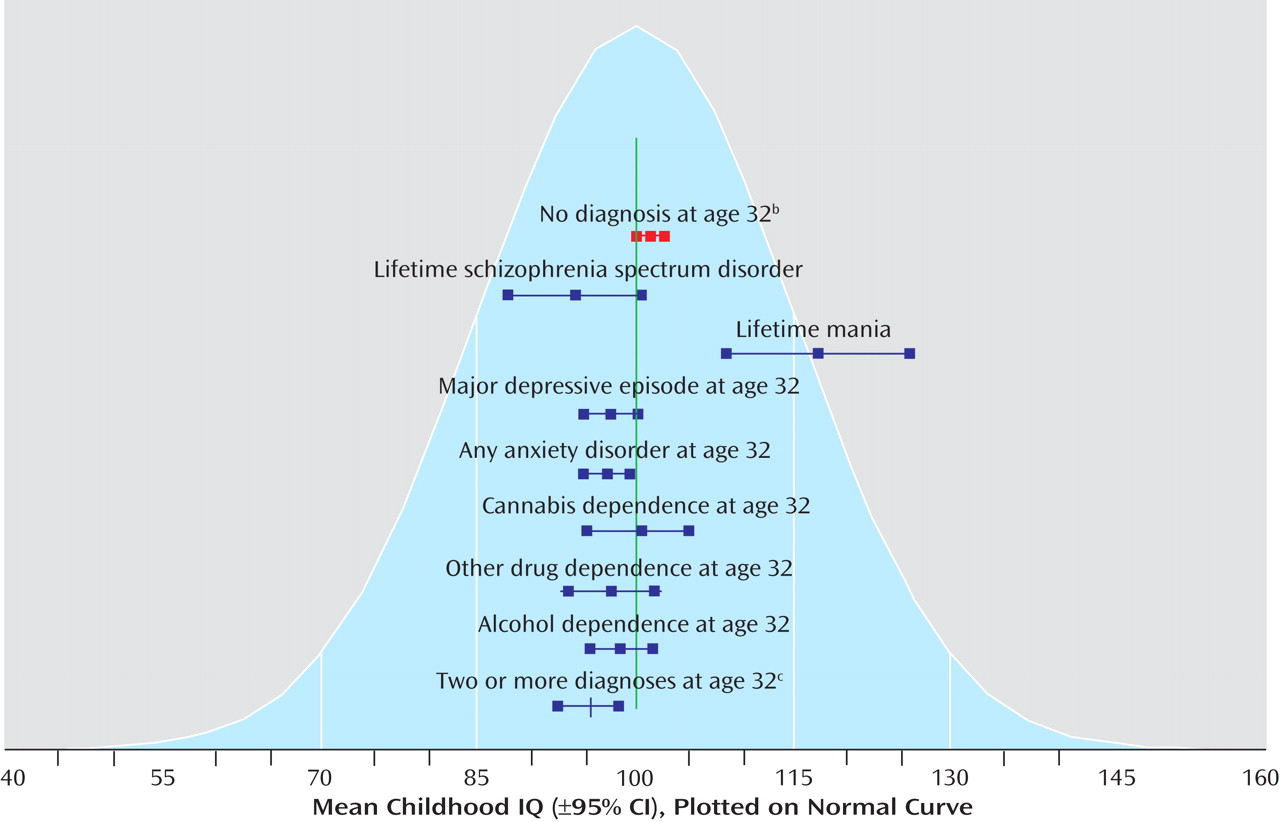
Figure 1 presents the mean childhood IQs and 95% CIs for the healthy comparison subjects, participants with specific psychiatric disorders, and those with disorders in two or more diagnostic families.
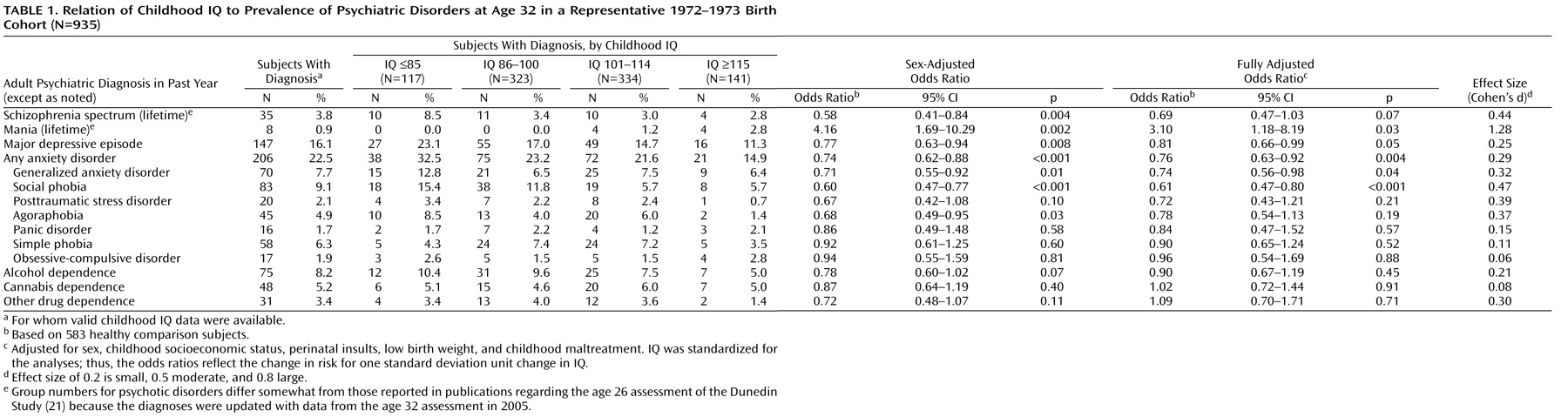
Table 1 presents the proportions of participants with specific diagnoses by childhood IQ categorized according to standard deviation units (15 points) from the mean. For each standard deviation increase in childhood IQ, the participants had a 42% (95% CI: 16%–59%) reduction in the odds of a lifetime schizophrenia spectrum diagnosis, a 23% (95% CI: 6%–37%) reduction in the odds of an adult depression diagnosis, and a 26% (95% CI: 12%–38%) reduction in the odds of an adult anxiety disorder diagnosis. These results remained significant after adjustment, although the finding for schizophrenia spectrum disorder became marginal with the addition of statistical controls for confounders. IQ was not associated with alcohol, cannabis, or other drug dependence. In contrast, higher IQ increased the risk of mania.
For specific anxiety disorders, higher childhood IQ predicted a 29% (95% CI: 8%–45%) reduction in the odds of having generalized anxiety disorder and a 40% (95% CI: 23%–53%) reduction in the odds of social phobia. Higher IQ also appeared to reduce the risk of PTSD and agoraphobia. These effects were nonsignificant owing to the small number of cases, but the effect sizes for these diagnoses were similar to the significant effect sizes for major depression and generalized anxiety disorder. The effect sizes for the predictive associations between childhood IQ and adult psychiatric disorders were small to moderate, except for mania, which had a large effect size (
Table 1, last column).
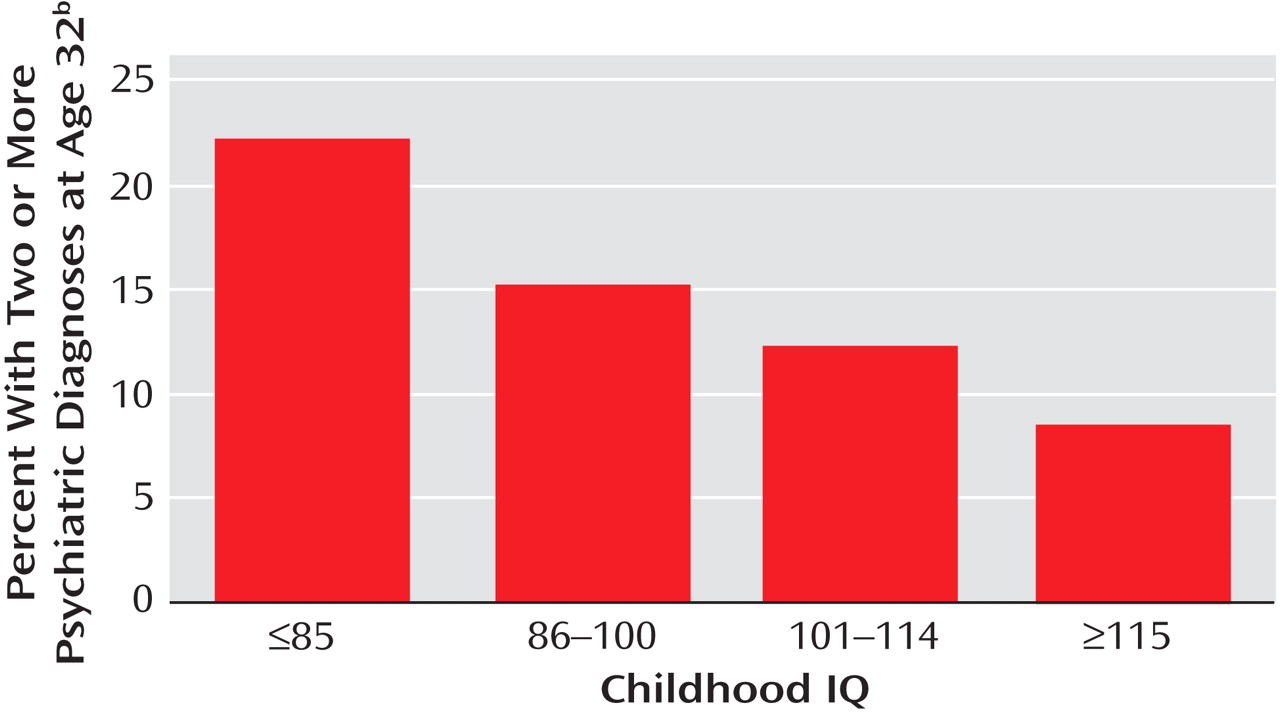
Figure 2 presents the proportion of participants with comorbid disorders in two or more diagnostic families at age 32 in childhood IQ groups categorized according to standard deviation units from the mean. Each standard deviation unit increase in IQ was associated with reduced risk of psychiatric comorbidity (odds ratio: 0.67, 95% CI: 0.55–0.82, p<0.001) in a dose-response fashion. The effect of IQ on comorbidity remained significant after adjustment for potential confounders (odds ratio: 0.74, 95% CI: 0.60–0.92, p=0.006). The effect size for the association between childhood IQ and comorbidity was 0.36.
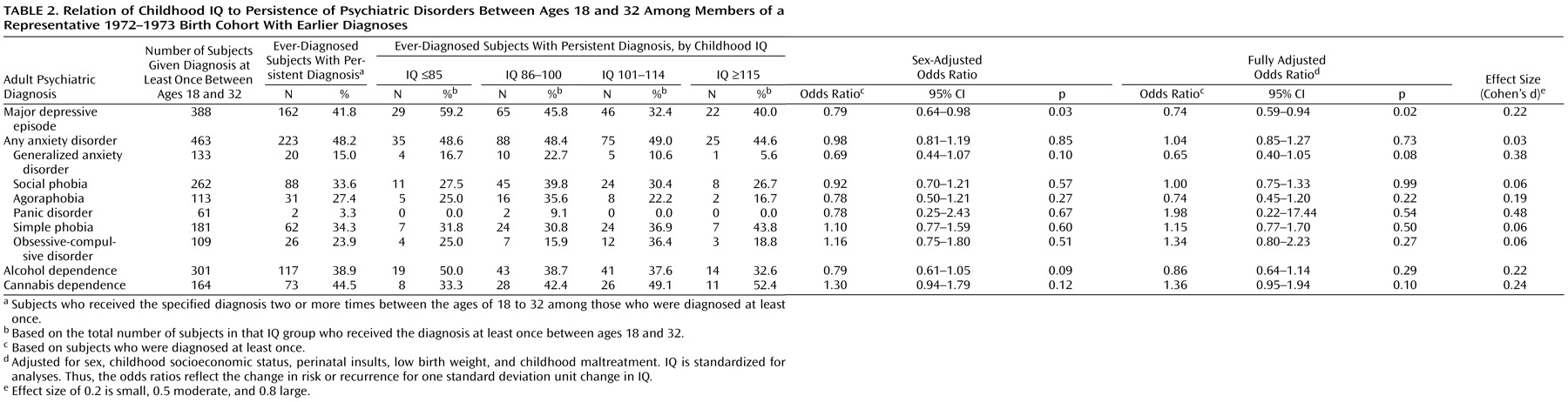
Table 2 presents the predictive association between childhood IQ and disorder persistence. Each standard deviation increase in IQ was associated with significantly reduced risk of persistence for depression. The association between higher IQ and less persistent generalized anxiety disorder was not statistically significant owing to the small number of cases (N=20) of persistent generalized anxiety disorder between ages 18 and 32.
At age 11, which overlapped with one administration of the WISC-R, 66 children had diagnosed depression or anxiety . To ensure that these childhood disorders did not confound our findings by interfering with IQ testing, we reanalyzed the data using the IQs at ages 7 and 9. The findings were consistent with those already presented.
Discussion
Lower childhood IQ predicted significantly increased risk of a diagnosis of schizophrenia spectrum disorder, major depression, or any anxiety disorder in adulthood. Lower IQ was not linked to adult substance dependence disorders. In contrast, higher, not lower, childhood IQ was significantly associated with mania, although only eight cohort members received this diagnosis. This finding for mania warrants replication, because it is counter to the cognitive reserve hypothesis, and thus it may have resulted from chance. Also, this finding is relevant to the debate about whether schizophrenia and mania are etiologically distinct. In addition, as predicted by the theory of cognitive reserve, lower childhood IQ was associated with greater comorbidity at age 32 and with more persistent major depression from 18 to 32 years of age; the association with more persistent generalized anxiety disorder was nearly significant. These associations were not confounded by sex, childhood socioeconomic status, perinatal insults, low birth weight, or childhood maltreatment.
To our knowledge, this is the first report of the association between childhood IQ and the full range of adult anxiety disorders. We found no association between childhood IQ and either the prevalence or persistence of simple phobia, panic disorder, or OCD. In contrast, lower childhood IQ was a risk factor for generalized anxiety disorder and social phobia, and lower IQ appeared to predict increased risk of PTSD and agoraphobia, although these associations were not significant because of the small number of participants with these diagnoses. Previous analyses of data from this cohort support the predictive association between lower childhood IQ and subsequent PTSD; lower IQ predicted the 96 cases of lifetime PTSD up to age 26 and new cases of PTSD between the ages of 26 and 32
(15) .
Childhood IQ was not associated here with a diagnosis of alcohol, cannabis, or other drug dependence at age 32, a result consistent with findings in at least two other prospective studies
(11,
12) . Moreover, childhood IQ did not predict persistent alcohol or cannabis dependence. The lack of an association between childhood IQ and the substance dependence diagnoses in our fully adjusted models suggests that any previously observed associations may have been due to confounding factors.
Limitations
This study has four main limitations. First, since persistence was based on a cumulative count of diagnoses, each of which was ascertained in a past-year assessment at ages 18, 21, 26, and 32, gaps between our past-year assessments may have led us to undercount some cohort members’ episodes of disorder. However, with this prospective approach, disorders are not undercounted because of participants’ failure to recall criterion symptoms from years past, as occurs in retrospective surveys
(28) . Moreover, we have shown that there is little case undercounting as a result of the gaps between past-year assessments. For example, only eight cohort members who had received mental health services for depression or anxiety in the years between assessments had not been diagnosed by the study’s repeated psychiatric interviews; no cases of psychosis, suicide, or substance dependence were missed. Second, our study to date has followed participants only through age 32. Further research must determine whether childhood IQ predicts disorders in older groups. Third, although we tested a directional hypothesis and emphasized effect sizes over p values, our comparison across multiple disorders required multiple statistical tests, and thus findings should be replicated. Fourth, results of the present study are limited to a single, contemporary cohort of New Zealand young adults. Further research is required to determine whether our findings will generalize to other times and places.
Conclusions
Our findings are consistent with the theory that lower cognitive reserve, as operationalized by childhood IQ, is a risk factor for major depression and certain anxiety disorders, with greater comorbidity and persistence of disorder. The mechanism through which low childhood IQ might confer these risks is unknown. We posit four mechanisms that warrant research. These are probably not mutually exclusive.
First, lower childhood IQ may be a marker of neuroanatomical deficits that increase vulnerability to certain mental disorders. Imaging studies of intelligence and brain structure and function reveal regions implicated in psychiatric conditions
(29) . For example, IQ is positively correlated with cerebellar volume
(30), and verbal IQ has been shown to correlate with hippocampal volume in male children
(31) . Smaller white matter and hippocampal volumes have been associated with depression and anxiety in correlational studies
(32,
33) .
Second, lower childhood IQ may be associated with adult psychiatric disorders through psychosocial stress. Individuals with lower childhood IQs are less equipped to cope with stressful life events, making them potentially more vulnerable to developing disorders after such events. Stressful life events are important in the etiology of major depression, generalized anxiety disorder, PTSD, and social phobia in particular
(34,
35) . Lower childhood IQ may also be a marker of deficits in cognitive functioning that make activities of daily living more challenging and, therefore, stressful. Daily activities common in our modern society, such as negotiating complicated public transportation systems, completing income tax forms, comparison shopping, managing money, helping children with homework, and navigating the Internet are more demanding for individuals with lower IQs
(36) . Individuals with lower IQs also tend to obtain low-status, low-skilled jobs
(37), which involve working conditions that have been shown to increase the risk of depression and anxiety in the Dunedin cohort
(38) .
Third, the association between childhood IQ and adult psychiatric disorders may be mediated by mental health knowledge. Mental health literacy is posited to reduce risk and improve outcomes for psychiatric disorders by facilitating early help-seeking, improving access to evidence-based care, and promoting treatment compliance
(39) . IQ has been shown to be the major determinant of health knowledge
(40) . Individuals with lower IQs are likely to have worse mental health literacy.
A fourth possibility is that IQ has little causal effect on mental health but that, rather, lower IQ is an antecedent to certain mental disorders because it shares some common etiology with them. For example, twin studies have shown that a common genetic liability accounts for part of the association between cognitive ability and posttraumatic stress disorder
(14) . We cannot determine from our data here whether the association between lower IQ and adult mental disorders is environmentally or genetically mediated.
Given that lower childhood IQ predicted greater psychiatric comorbidity in this study, it seems likely that many individuals who seek mental health treatment may also have lower cognitive ability. Patients with lower cognitive ability could have difficulty accessing services or difficulty understanding and complying with treatment protocols. These individuals may benefit from interventions aimed at improving mental health literacy. Cognitive ability may, therefore, be important to consider in prevention and in treatment planning.