The prevalence of depression in children and adolescents is on the rise, and depressive illness in these developmental periods is associated with significant impairment in multiple social domains. Elevated risk for the disorder begins in the early teens and continues to rise in a linear fashion throughout adolescence, with lifetime rates estimated to range from 15% to 25% by late adolescence
(1,
2) . There is evidence that early depressive episodes recur and persist into adult life along with ongoing psychosocial difficulties, including disruption in interpersonal relationships, early pregnancy, low educational attainment, poor occupational functioning, and unemployment, as well as elevated risk for suicidal behavior
(2) .
Depression in youth may be complicated by substance abuse and may be a precursor to substance use disorder in some youngsters
(3,
4) . When these two disorders occur together, adaptive function is compromised further. The co-occurrence of substance use disorder with depression in adolescents is associated with earlier onset and more severe substance-related problems, increased frequency of behavioral problems, more prolonged and recurrent depressive episodes, and more severe impairment in family, school, and legal domains
(4) . Youth with the comorbid disorder also are at higher risk for suicidal behavior than those without comorbidity
(4) . As a result, the comorbid illness is associated with increased use of health services and substantially higher treatment costs compared with those who have only depression or substance use disorder
(5) . Given the economic and psychosocial burden associated with comorbid depression and substance use disorder, understanding the underlying mechanisms associated with these comorbid conditions is of great public health importance.
The theory that psychosocial stress is associated with depression has been studied extensively, and there is substantial evidence for the contribution of stressful life experiences in precipitating depressive episodes
(6) . Psychosocial stress is postulated to be an important factor in the initiation and maintenance of substance use disorder as well. Various hypotheses describe stressors as cues that elicit anticipatory alcohol/drug use responses, as stimuli that evoke negative affective states and prompt substance use to alleviate this emotional distress, or as events that place adaptational demands on an individual
(7 –
9) .
Although studies on depressive illness and substance use disorder emphasize the role of stress in the initiation and maintenance of these disorders, there are individual differences with respect to the impact of stress on these disorders. Research in animals and humans suggests that an increased response of the brain stress systems (of which the hypothalamic-pituitary-adrenal [HPA] axis is a component) moderates the effects of stress exposure on drug-seeking behavior
(10,
11) . These stress systems are stimulated by acute drug administration and are known to activate mesolimbic dopamine pathways that are important in the rewarding properties of addictive drugs
(7,
10) . Alterations in the HPA system also have been reported in numerous investigations of depression
(12,
13) . Data from preclinical and clinical studies suggest that the sensitization of the HPA axis to stress may be more prominent during the early developmental stages than in adulthood, indicating that early onset of depressive illness has the potential for significant added long-term negative consequences
(12,
14,
15) .
In a longitudinal study, we found that stressful experiences precipitated substance use disorder episodes in adolescent females
(16) . In a separate investigation, we reported that higher cortisol secretion near bedtime, a time when the HPA axis is normally quiescent, was associated with increased risk for the development of substance use disorder in depressed adolescents
(17) . The present work extends these findings by combining both of these measures in a new cohort of depressed and nondepressed youth. Development of substance use disorder was assessed prospectively with systematic assessments at regular intervals. Furthermore, the association between substance use disorder and depression during follow-up was evaluated. It was hypothesized that depressed adolescents would be at greater risk for developing substance use disorder during follow-up than their nondepressed counterparts. Reciprocally, substance use disorder would be associated with higher frequency of depressive episodes. Adolescents who develop substance use disorder would have higher HPA activity at intake, and recent stressful experiences would moderate the relationship between HPA activity and vulnerability to substance use disorder, independent of depression history.
Method
The original study was focused on the development and recurrence of depression in adolescents and potential predictors of clinical course, with emphasis on HPA activity, sleep polysomnography, and stressful life events. Based on prior literature and data from our laboratory, the development of substance use disorder also was incorporated into the protocol.
Participants
The participants for the study included 55 adolescents with depression, 48 adolescents at high risk for developing depression by virtue of parental depression (high-risk comparison subjects), and 48 normal comparison subjects. Depressed subjects met criteria for major depressive disorder based on the DSM-IV
(18), with a minimum duration of 4 weeks and a score of ≥15 on the first 17 items of the Hamilton Depression Rating Scale (HDRS)
(19) . Adolescents with a current or prior history of mania/hypomania, psychotic disorder, or substance use disorder symptoms were excluded from the study. Only experimental substance use was allowed; youth with lifetime history of ≥2 times/week of alcohol/illicit drug use for ≥4 weeks or any use in the past month were excluded. Subjects also were excluded if there was a family history of bipolar disorder. Participants were free from psychotropic agents for at least 8 weeks. The normal and high-risk comparison subjects were free from psychopathology in their lifetime. In addition, the high-risk comparison subjects had at least one biological parent with a history of unipolar major depression that required treatment. Normal comparison subjects were not included if any first-degree relative had history of a psychiatric disorder. Participants were medically healthy and free from alcohol/illicit drug use, as determined by physical examination, full chemistry panel, thyroid function tests, electrocardiogram, and urine drug screen.
Diagnostic Evaluation
The diagnosis of major depressive disorder and other psychiatric disorders was based on a semistructured instrument, the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL). Interrater and test-retest reliability have been established for this instrument and have convergent and discriminant validity
(20) . The K-SADS-PL was administered separately to the adolescent and parent, and summary scores were tabulated. The Children’s Global Assessment Scale (CGAS), a global psychosocial functioning measure, also was completed
(21) . The adolescent participants completed the Beck Depression Inventory (BDI) for self-assessment of depression severity
(22) .
The Family History Research Diagnostic Criteria (FH-RDC), a semistructured instrument, was used for the evaluation of psychiatric disorders in family members
(23) . A parent was interviewed regarding major psychiatric disorders over the lifetime in all first-degree relatives of the adolescent subject. The FH-RDC is a sensitive instrument for obtaining information on psychopathology from knowledgeable relatives.
Stressful Life Experiences
Using the contextual threat method
(24), participants were probed systematically about the occurrence and timing of particular life events, as well as details of the context in which the events occurred
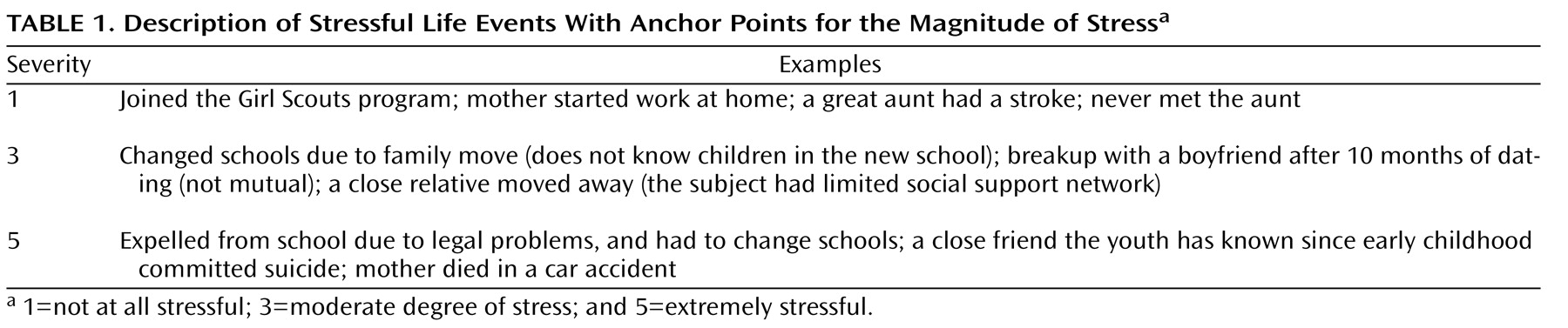
(25) . For the baseline assessment, the time frame for events included the previous 6 months. Narrative summaries of the events were presented to a group of trained raters. The raters were blind to the participant’s diagnostic status and reaction to the stressor. Consensus group ratings were given for the degree of stress for each event (1=not at all stressful, and 5=extremely stressful) and whether the event was a positive, neutral, or negative experience under the given circumstances. Symptom-related events were not scored. Only events that were considered negative were included in the analyses. Examples of some life events with different degrees of stress are described in
Table 1 . Good interrater reliability has been established for the episodic stress ratings
(25) .
HPA Measures
Each subject participated in a 3-night sleep-neuroendocrine study in the laboratory. Prior to these studies, sleep-wake schedules were regulated for at least 1 week, with participants going to bed between 10:00 p.m. to 11:00 p.m. and waking between 6:30 a.m. and 7:30 a.m. The sleep-wake schedule was confirmed through diary and actigraphy. The participants were free from alcohol/illicit drug use at the time of HPA studies (confirmed by urine drug screen test). Subjects were asked to void urine before switching off the lights. All urine voided during the night (including the sample immediately upon awakening) was collected. A radioimmunoassay procedure was employed for the cortisol assay
(26) . Low, medium, and high cortisol pools were reanalyzed in each assay to assess intra- and interassay variability. The intra- and interassay coefficients of variation for the assays were less than 10%. Samples from the same subject were analyzed in the same assay.
Follow-Up Evaluation
After the baseline assessments, the participants were followed longitudinally at 6-month intervals in the first year and yearly thereafter, for up to 5 years, to obtain information on the clinical course of depression and other psychiatric disorders (also including the onset of substance use disorder). Substance use disorder diagnosis was based on DSM-IV criteria for either abuse or dependence
(18) . Development of a depressive episode was defined as a rating of 5 on the Psychiatric Status Rating (PSR) component of the Longitudinal Interval Follow-Up Evaluation (LIFE) for minimum 4-week duration. Remission from a depressive episode was defined as a rating of 2 for ≥12 weeks on the PSR. Recurrence was defined as a PSR rating of ≥5 for 4 or more weeks with a minimum duration of 12 weeks between episodes. The LIFE is a semistructured instrument used for charting the clinical course of depression and other psychiatric disorders during longitudinal follow-up
(27) . The PSR is a 6-point scale providing information on the severity of depressive symptom profile.
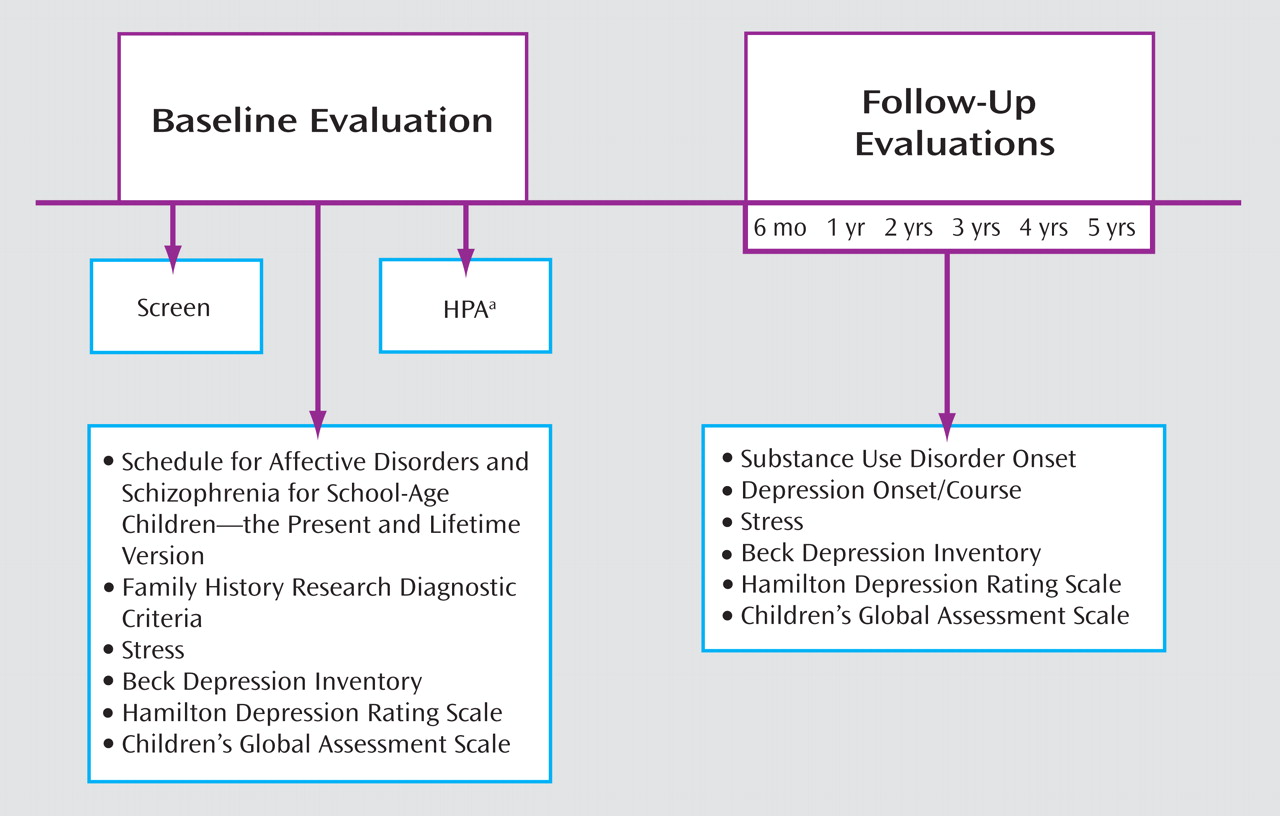
Information from the diagnostic assessments was presented to an independent clinician “blind” to the diagnostic status, stressful life events, and HPA status. Final diagnosis was based on consensus ratings. Assessment of stressful experiences during the follow-up period was similar to the method used during initial evaluation. Time frame for the events included the period since the last interview. Ratings for the magnitude of stress occurred blind to information from the diagnostic and HPA assessment. A summary of the baseline and follow-up assessments is depicted in
Figure 1 .
Primary Dependent and Independent Variables
The primary outcome measure was onset of substance use disorder during follow-up. The primary independent variables included nocturnal urinary-free cortisol concentration and recent stressful experiences. Timelines were generated, charting the onset of substance use disorder and stressful life events. For each participant who developed substance use disorder during the follow-up period, the total stress score for the 3 months preceding the onset of substance use disorder was computed. Each subject who did not develop substance use disorder was paired with a participant with substance use disorder based on demographic information, and stressful events that he/she experienced in the corresponding 3 months were tabulated. This method was adopted instead of using a random 3-month period because events were not evenly distributed across the 5 years owing to developmentally expected events (e.g., school transitions). For nocturnal urinary-free cortisol, a single mean value was obtained across the 3 nights. Secondary outcome measures included onset or recurrence of a depressive episode during follow-up. Substance use disorder was the independent measure in these analyses.
Data Analytic Plan
For group comparisons, the chi-square was used to analyze categorical variables and analysis of variance for continuous variables. Correlation procedures were used to examine associations between variables. Cox regression, with appropriate covariates, was used to compute the probability of developing substance use disorder during follow-up. Stress score in the 3 months preceding the onset of substance use disorder, nocturnal urinary-free cortisol, and the interaction term were used as independent variables.
Results
Sample Characteristics
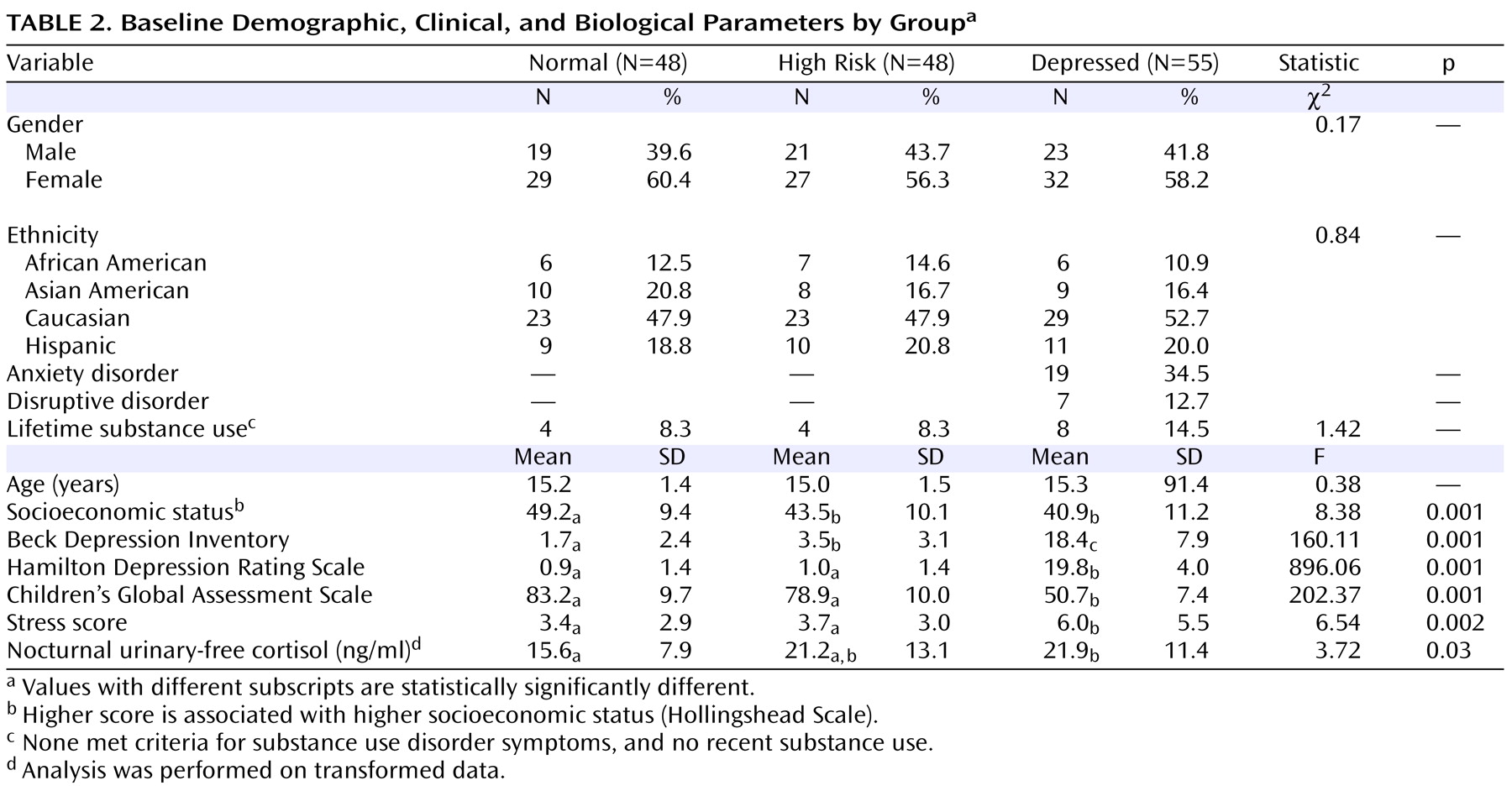
Demographic, clinical, and nocturnal urinary-free cortisol variables in the three groups of adolescents at the time of initial evaluation are outlined in
Table 2 . The groups did not differ significantly with respect to age, gender, or race/ethnicity. High-risk and depressed groups had significantly lower socioeconomic scores than normal comparison subjects. Depressed adolescents scored significantly higher on the BDI, HDRS, and stressful experiences, but lower on CGAS, than both comparison groups. The groups did not differ significantly with respect to experimental alcohol/drug use before recruitment. Depressed youth had significantly higher mean nocturnal urinary-free cortisol concentration than normal comparison subjects, whereas the high-risk subjects had intermediate levels.
Follow-Up Information
Three initially normal comparison subjects, four high-risk comparison subjects, and four depressed adolescents did not have any follow-up evaluations. Subjects who did not participate in follow-up assessments did not differ significantly from those with follow-up information on any demographic or clinical characteristics. Of the 140 adolescents who had follow-up information, 9.3% were followed for 2 years, 19.3% for 3 years, 31.4% for 4 years, and 40.0% for 5 years. The three groups were comparable on the mean follow-up interval (mean follow-up interval=3.6 years, SD=1.0).
Development of Substance Use Disorder During Follow-Up
Four initially normal comparison subjects (4 of 45, 8.9%), 10 of 44 (22.7%) adolescents initially identified as being at high risk for depression, and 19 of 51 (37.2%) initially depressed adolescents developed substance use disorder during follow-up (χ 2 =10.70, df=2, p≤0.005). Among the 33 subjects that developed substance use disorder, 15 (45.4%) had only alcohol use disorder and six met criteria for dependence, 9 (27.3%) had illicit drug use disorder and four met criteria for dependence, and 9 (27.3%) had both alcohol and drug use disorder and three met criteria for dependence. Among illicit drugs, marijuana abuse was most common (55.6%), followed by stimulants (22.2%) and opiates (16.7%). Information on the developmental progression of substance use disorder as well as demographic and clinical predictors of substance use disorder is provided in the data supplement (available at http://ajp.psychiatryonline.org).
Effects of HPA Activity and Stressful Experiences on Vulnerability to Substance Use Disorder
Age, gender, ethnic background, socioeconomic status, depressive symptoms, and family history of depression did not correlate significantly with nocturnal urinary-free cortisol or magnitude of stress preceding substance use disorder. Stress at intake did not correlate significantly with nocturnal urinary-free cortisol (r=0.14). There was, however, a modest but significant correlation between stress at intake and stress preceding substance use disorder (r=0.26, df=140, p≤0.005), and between nocturnal urinary-free cortisol and stress preceding substance use disorder (r=0.24, df=140, p≤0.005). After control was added for the effect of stress at intake, the correlation between nocturnal urinary-free cortisol and stress preceding substance use disorder persisted (r=0.20, df=137, p≤0.05).
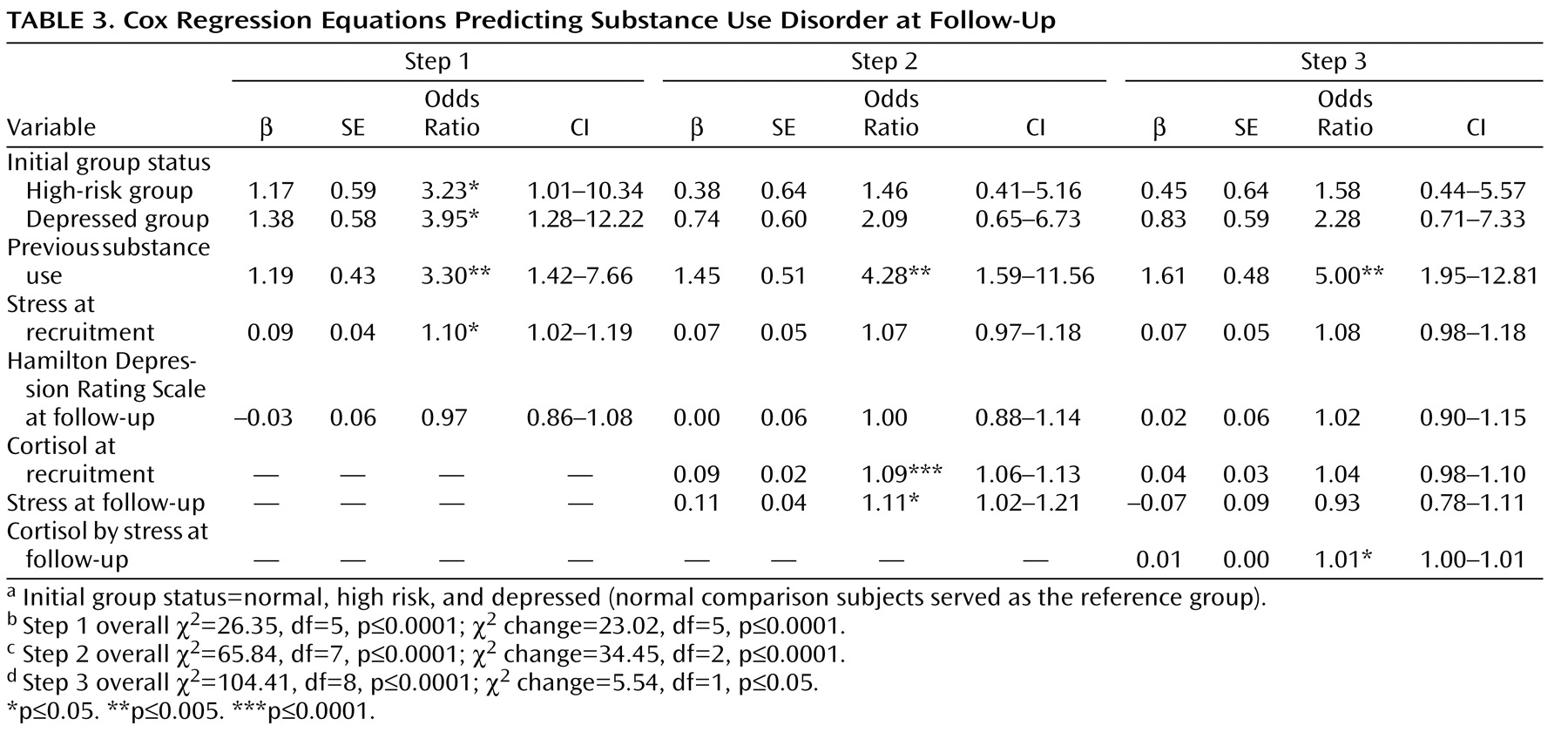
Although depression status at intake (normal versus high risk versus depressed) increased the risk for substance use disorder (see Step 1 in
Table 3 ), the effect of depression on substance use disorder was attenuated when nocturnal urinary-free cortisol and stressful experiences were added in the model (see Step 2 where high risk and depressed status were no longer significant). After control was added for the effects of depression, lifetime substance use, and stress at intake, the combination of nocturnal urinary-free cortisol and stress at follow-up (nocturnal urinary-free cortisol by stress) predicted substance use disorder although the effect was modest (see Step 3 in
Table 3 ).
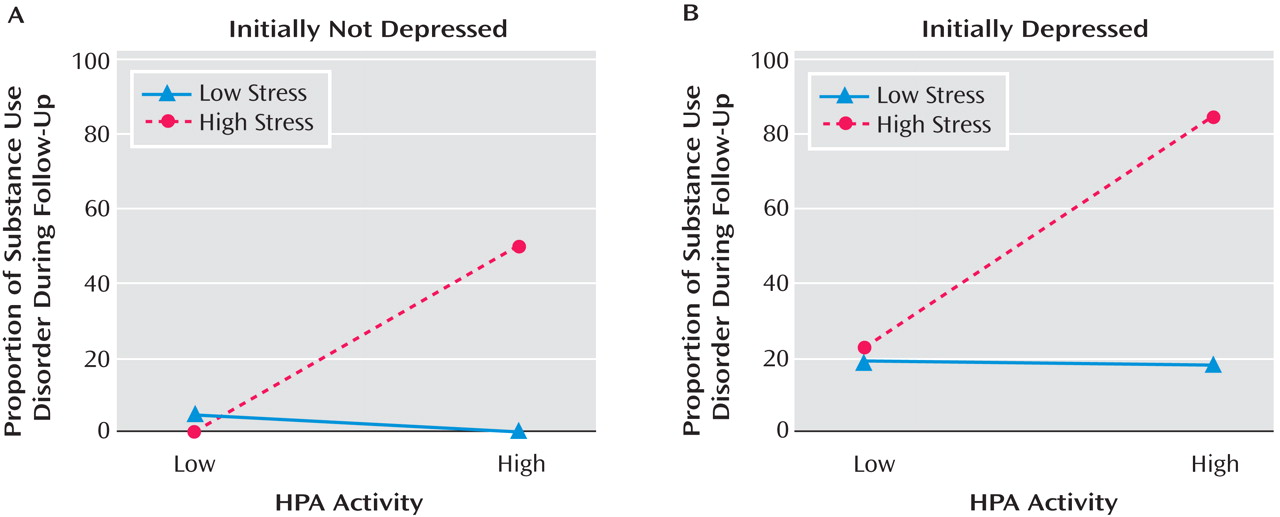
A graphical representation of the effects of nocturnal urinary-free cortisol and stress on vulnerability to substance use disorder in the initially normal and high-risk subjects is provided in
Figure 2 A. Subjects were reclassified based on HPA activity and stress levels. HPA activity was dichotomized based on a median split: low HPA activity (N=45) and high HPA activity (N=44). Within the low HPA activity group, one subject (2.2%) developed substance use disorder, whereas 13 of 44 (29.5%) from the high HPA activity cohort developed the disorder (χ
2 =12.53, df=1, p≤0.0001). The sample was then substratified based on stress scores. In the low HPA activity group, one of 25 (4.0%) subjects with low stress level developed substance use disorder compared to none from the high stress (N=20) group (Fisher’s exact, n.s.). In the high HPA activity group, none with low stress level (N=18) developed substance use disorder compared to 13 of 26 (50.0%) of the high stress group (Fisher’s exact, p=0.0001; see
Figure 2 A).
Using the same procedure in initially depressed adolescents, five of 25 (20.0%) youth from the low HPA activity group developed substance use disorder compared with 14 of 26 (53.8%) from the high HPA activity cohort (χ
2 =8.62, df=1, p≤0.05). Within the low HPA activity group, three of 16 (18.8%) subjects with low stress level developed substance use disorder, whereas two of nine (22.2%) youth from the high stress group developed the disorder (Fisher’s exact, n.s.). In the high HPA activity cohort, two of 11 (18.2%) with low stress level developed substance use disorder compared to 12 of 15 (80.0%) in the high stress group (χ
2 =9.76, df=1, p≤0.005; see
Figure 2 B).
These data suggest that the effects of HPA activity and stressful life experiences on vulnerability to substance use disorder are independent of depression status at baseline.
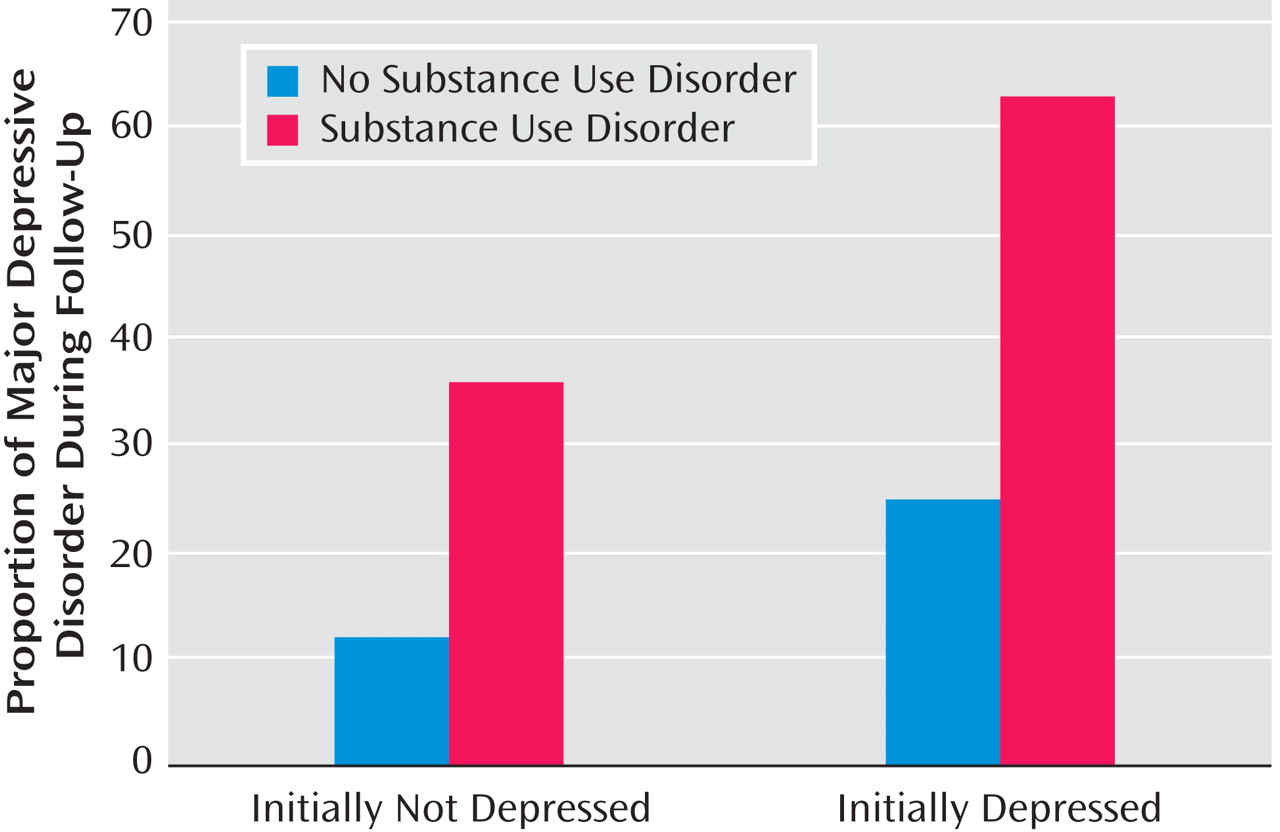
Relationship Between Substance Use Disorder and Depression During Follow-Up
Of 45 initial normal comparison subjects with follow-up information, four (8.9%) developed major depressive disorder during the study period. Also, 10 of 44 (22.7%) adolescents initially identified as being at high risk for depression developed major depressive disorder. Combining both groups, a higher proportion of participants with substance use disorder developed major depressive disorder during follow-up compared with their counterparts without substance use disorder (Fisher’s exact, p≤0.05; see
Figure 3 ). Of the five initial normal or high-risk subjects who developed both major depressive disorder and substance use disorder episodes during follow-up, substance use disorder preceded depression in two youngsters and depression preceded substance use disorder in three. Of 51 initially depressed adolescents who had follow-up evaluation, 20 (39.2%) developed a recurrent depressive episode. A higher proportion of depressed youth with substance use disorder had a recurrent depressive episode compared with those who did not develop substance use disorder (χ
2 =5.77, df=1, p≤0.05; see
Figure 3 ). Of the 12 initially depressed subjects who had both major depressive disorder and substance use disorder episodes during follow-up, substance use disorder preceded recurrent depressive episodes in six youngsters, recurrent depression preceded substance use disorder in four youth, and both were concurrent in two youngsters.
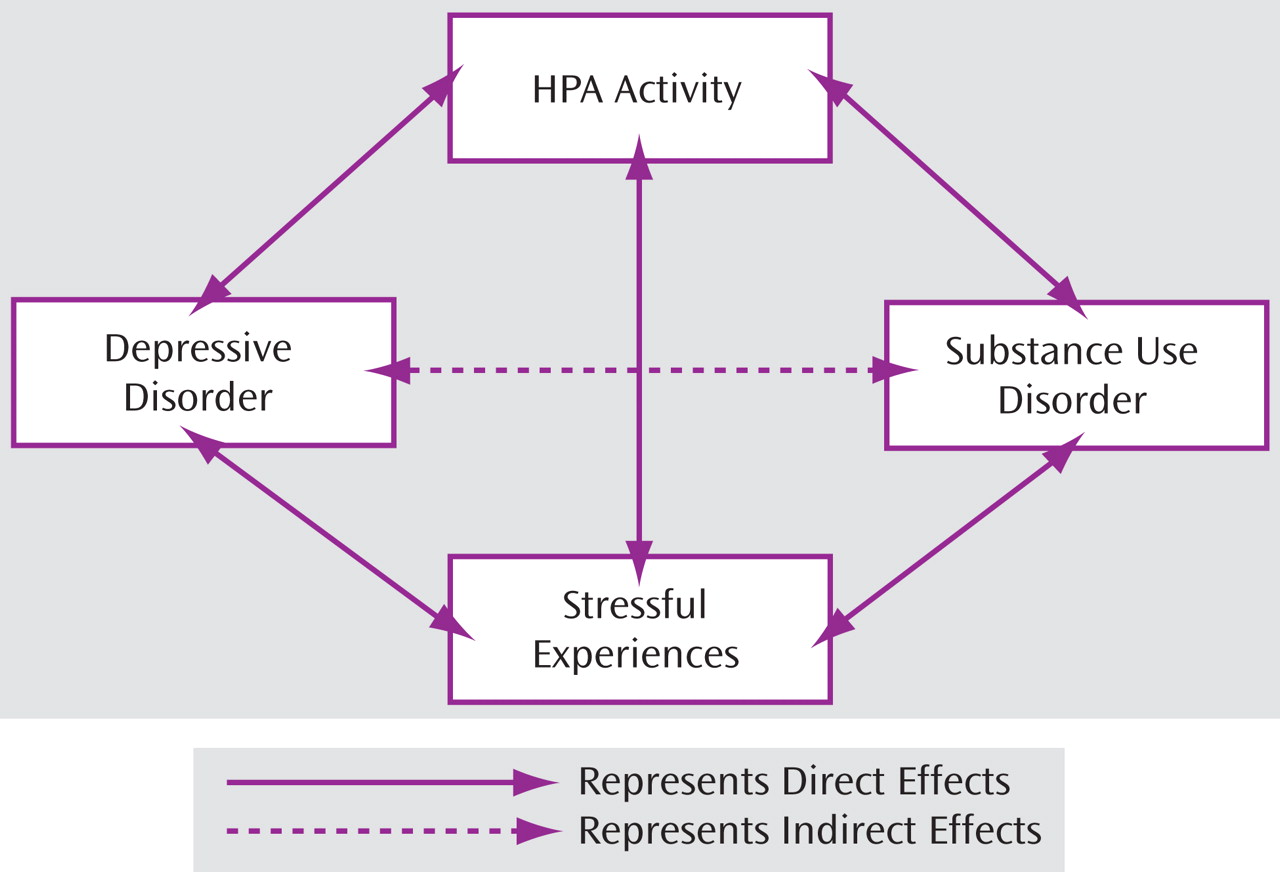
Underlying Mechanisms Associated with Comorbid Depressive and Substance Use Disorders
Based on the results presented in the preceding two sections, a conceptual model of the relationship between depression and substance use disorder is proposed (see
Figure 4 ). Depression is associated with increased risk for substance use disorders in adolescents and adults compared to their counterparts without depression. The increased risk for substance use disorders in depressed individuals might be mediated or moderated, in part, by the elevated HPA activity and higher stress levels frequently observed in these populations. Reciprocally, substance use disorder increases vulnerability to depressive episodes indirectly through its effects on HPA activity and stressful life experiences.
Discussion
To the best of our knowledge, this is the first study to integrate prospectively both neurobiological and psychosocial factors as vulnerability markers for substance use disorder in depressed and nondepressed adolescents. Consistent with our previous finding, adolescents who developed substance use disorder had higher HPA activity at intake than those who did not develop substance use disorder
(17) . However, the relationship between elevated HPA activity and substance use disorder was influenced by stressful experiences such that only those adolescents who experienced high levels of stress during prospective follow-up were more likely to develop substance use disorder
(16) . As demonstrated in several nonreferred adolescent samples, the frequency of substance use disorder was higher in depressed youth compared to their counterparts without depression
(3), and the co-occurrence of depression and substance use disorder was demonstrated prospectively as well
(16) . However, the relationship between depression and substance use disorder was attenuated when HPA dysregulation and stressful experiences were accounted for, suggesting that their association may be explained, at least in part, by these factors
(4,
6,
28) .
These results should be considered in the context of study limitations. First, the small number of adolescents with substance use disorder precludes in-depth analysis of the substance use disorder subtypes. Due to the lack of a substance use disorder group at baseline, it was not possible to systematically assess the reciprocal relationship between depression and substance use disorder. Despite the statistical significance, the combined effect of nocturnal urinary-free cortisol and stress on substance use disorder was only modest, and the follow-up interval was relatively short. Although elevated HPA activity was associated with vulnerability to substance use disorder
(17), another study reported lower cortisol response to an anticipated stressor in preadolescent boys whose biological fathers had substance use disorder
(29) . In this cohort, the lower cortisol response during preadolescence was associated with “regular” substance use during adolescence. Antisocial disorders mediated the risk for substance-related problems in the “high risk” group
(30) . In contrast, the elevated HPA activity in depressed adolescents in our studies was confounded by comorbid anxiety disorder (
17 ; also see online data supplement). The association between HPA dysregulation (lower versus higher activity) and vulnerability to substance use disorder may be influenced by the nature of psychopathology. Therefore, including a comparison group at high-risk for substance use disorder based on externalizing problems or familial risk would have been beneficial in clarifying these relationships. Also, it is important to note that HPA assessment was performed only at intake. Hence, a direct association between HPA activity and development of substance use disorder could not be assessed. Previous investigations showed that cortisol levels decline during remission from a depressive episode
(31) . Although clinical state influences HPA activity, evidence suggests that variation in HPA activity is heritable
(32) . High nocturnal urinary-free cortisol levels in a subgroup of high-risk controls before the development of any psychopathology also suggest the influence of heritability or the effects of being exposed previously to stressful situations.
Experimental studies in animals showed that corticosteroids increase self-administration of addictive substances
(7,
11) . Reciprocally, psychoactive substances reduce stress-induced alterations in corticosterone and adrenocorticotropin and attenuate the anxiogenic effect of corticotropin-releasing factor
(11,
33) . In humans, alcohol or cocaine consumption has been shown to reduce plasma adrenocorticotropin and cortisol responses to corticotropin-releasing hormone (CRH) or to induction of stress by various means
(10,
34) . Other studies indicated that acute withdrawal from addictive drugs is associated with activation of the HPA system
(10,
11) . It is postulated that these actions of psychoactive substances on the HPA system predispose individuals to initiate alcohol/drug use during stressful situations, finally leading to dependence. Consistent with this hypothesis, in an ongoing study of a group of adolescents with depression and/or nicotine addiction, depressed youth with nicotine addiction had significantly lower HPA response to a standard psychosocial stressor in the laboratory than their counterparts without smoking history, and also compared to smokers without depression history (Rao et al., unpublished data). Longitudinal assessment of HPA function in depressed adolescents before and after the development of substance use disorder will be helpful in clarifying whether chronic substance use downregulates the HPA system in this population.
The clinical implications of these findings are twofold. First, individual differences in HPA activity and their relation to substance use disorder vulnerability indicate a potential for the development of more specific interventions for the different subgroups and, ultimately, for an individual. For example, metyrapone, an inhibitor of corticosteroid synthesis, and CRH antagonists reduce self-administration of alcohol and cocaine in rodents
(35,
36) . Anti-glucocorticoid agents and CRH antagonists appear to have antidepressant properties and have been tested in humans for the treatment of depression
(13,
37) . Antidepressant agents also are helpful in reducing substance use, particularly in those with comorbid depression
(38) . However, some individuals with comorbid depression and substance use disorder show a poor response to antidepressant drugs
(38) . The differential response to antidepressant compounds in patients with substance use disorder might be related to HPA activity. It is postulated that persons with elevated HPA activity might benefit most from antidepressant agents. Data from clinical and preclinical studies suggest that treatment with antidepressant agents reduces responsivity to stress
(13,
39) . Second, the additional contribution of stressful life experiences in increasing the vulnerability to substance use disorder suggests that such persons might benefit from adjunctive psychosocial interventions
(40) . Future studies should evaluate the efficacy of pharmacotherapy and psychosocial interventions, singly and in combination, in patients with depressive and/or addictive disorders stratified on HPA activity and stress levels.