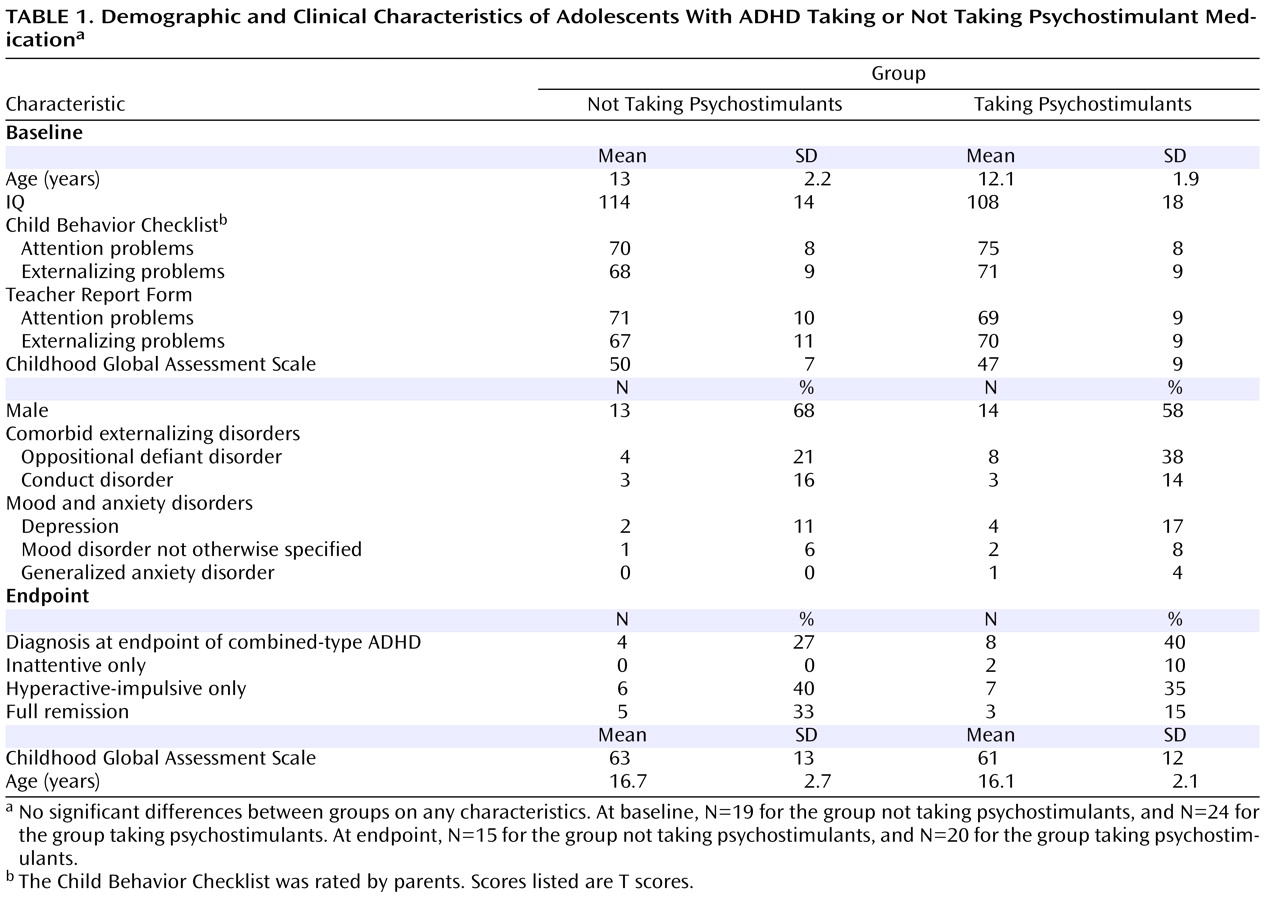
Psychostimulants represent the largest single class of psychotropic medication prescribed to children in the United States, with around 9% of all boys and 4% of girls receiving these medications for treatment of attention deficit hyperactivity disorder (ADHD)
(1) . The long-term safety of psychostimulants is thus of great importance
(2) . In two large randomized trials, psychostimulants were found to suppress growth rates during treatment, with a decrease of 1.3 cm per year in height and between 1.3 kg per year (for preschool age) and 2.5 kg per year (for school-age children) in weight from age-appropriate growth rates
(3,
4) . This finding raises the question of whether there might be similar effects on development of the brain.
A meta-analysis of neuroanatomic studies found that ADHD is characterized by reductions in gray and white lobar volumes, with some prefrontal cortical regions being particularly affected
(5) . However, there have been few studies of the structural correlates of psychostimulant treatment. Castellanos et al.
(6) found that prior psychostimulant treatment in children with ADHD was associated at study entry with greater white matter lobar volumes relative to those of stimulant-naive children with ADHD; volumes in the medication-treated group were also closer to the range of those in typically developing children, suggesting a neuroprotective effect of psychostimulants. Pliszka et al.
(7), in a study examining regions implicated in the pathogenesis of ADHD, similarly found that treatment with psychostimulants was associated with more normative volumes of the caudate and the anterior cingulate cortex
(7) . While informative, these studies were cross-sectional, limiting inferences that can be made about the developmental effects of medication, and they examined change only within a priori defined regions of interest or at the level of entire lobes.
We recently reported evidence of a delay in cortical maturation in ADHD in a study examining the age at which cerebral cortical points reached their peak thickness—that is, the point at which childhood cortical thickening gave way to thinning
(8) . Whereas typically developing children without ADHD reached peak cortical thickness in the frontal cortex around ages 7–8, in children with ADHD this developmental milestone was reached around ages 10–11. Throughout adolescence, both children with ADHD and healthy children show cortical thinning throughout nearly the entire cortex.
In this study, we examined whether treatment with psychostimulants affects these developmental trajectories. We selected a subset of patients from our cohort who underwent repeated neuroanatomic imaging and either received treatment with psychostimulants between scans or did not. We confined our examination to the age range of 9–20 years, which included most of those who were not taking psychostimulants during the interscan period. We compared the group not taking psychostimulants with both an age-matched group of ADHD youths who did receive psychostimulant treatment during the interscan period and a cohort of typically developing children without ADHD
(9) . All data thus lay within a period of cortical development predominately characterized by thinning. To our knowledge, this is the first prospective study examining whether cortical development reflects differing treatment with psychostimulants.
Discussion
Our results show no evidence that psychostimulants were associated with “slowing” of overall growth of the cortical mantle—a notable finding given reports of possible psychostimulant-related slowing of height and weight gain in children and adolescents
(3,
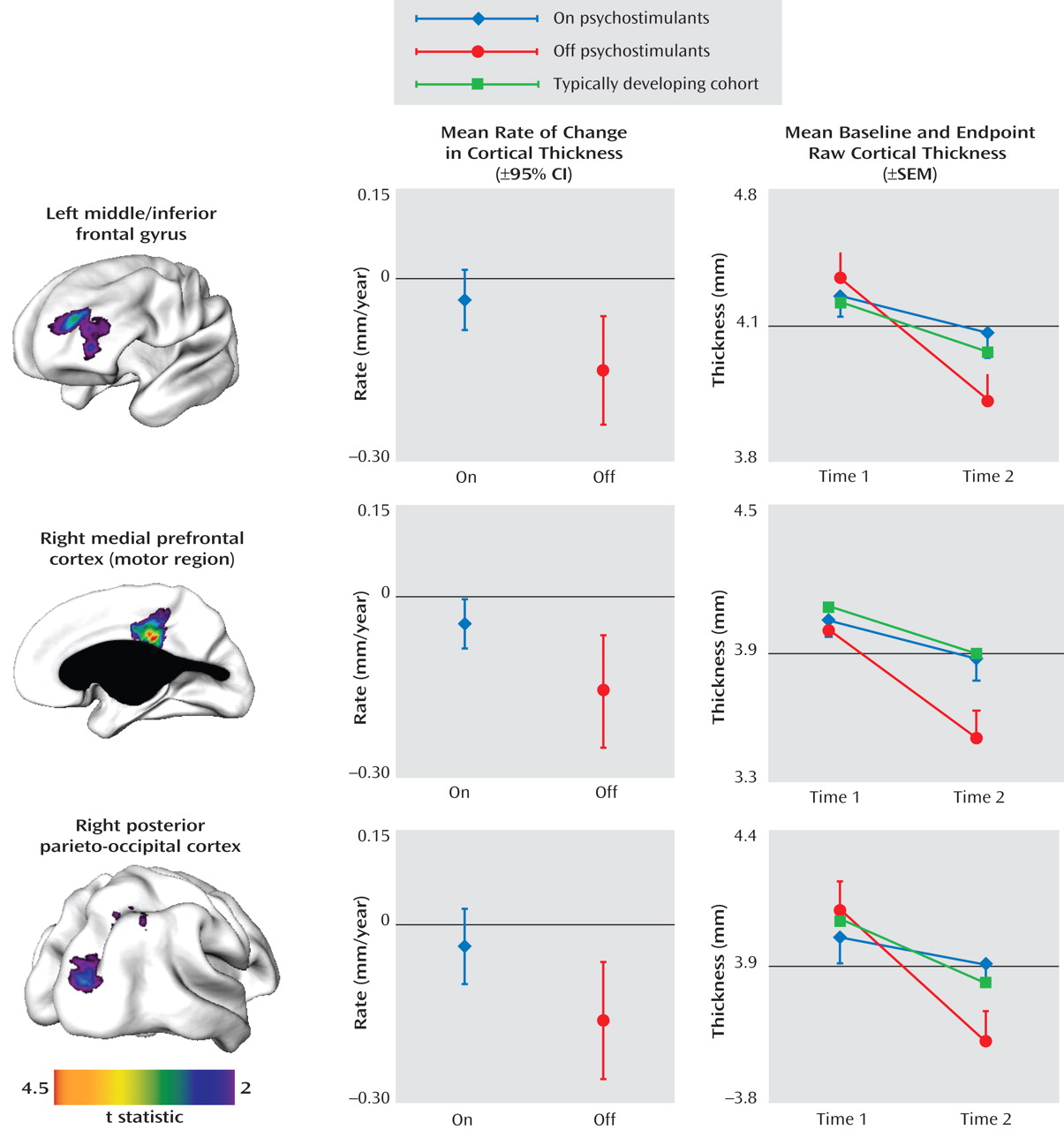
4) . Adolescents with ADHD who were not taking psychostimulants showed regional decreases in cortical thickness relative to both age-matched patients with ADHD who took psychostimulants and a typically developing cohort. The functional significance of this finding is unclear, however. We did not collect cognitive data at both time points in most participants. Also, the increased cortical thinning in the group that stopped taking psychostimulants was not associated with any difference in clinical outcome.
With these caveats in mind, it is worthwhile considering some possible interpretations. Psychostimulants tend to normalize goal-directed activity
(19 –
22) and cognitive processes, including planning, cognitive flexibility, vigilance, and response inhibition
(23) . In healthy adults, methylphenidate-induced improvement in working memory is associated with alterations of cerebral blood flow in the left dorsolateral prefrontal, supplementary motor, and posterior parietal cortex—overlapping in part with the regions we found to be differentially sensitive to psychostimulants
(24) . In children with ADHD, psychostimulant-induced improvement in the ability to inhibit prepotent responses is associated with increased frontal (and striatal) activity as assayed by functional MRI
(25) . In adults with ADHD, correction of executive deficits by psychostimulants is associated with altered prefrontal cortical activity—with increased activation of the premotor and decreased activation of the middle and medial prefrontal cortex
(26) . Thus, psychostimulant-induced increases in age-appropriate levels of cognition and action, and perhaps underlying localized frontoparietal neural activity, might foster cortical development within the normative range. In this regard, psychostimulant effects on the developing brain in ADHD can be conceptualized as an example of activity-dependent neuroplasticity. Additionally, it is possible that psychostimulants have a direct trophic effect on the cortex, particularly in view of the growing evidence for the role of catecholaminergic neurotransmitters in cortical development
(27,
28), although this explanation does not account for the highly regional effects detected in our study.
This study extends a previous demonstration of more normative white matter volumes in youths with a history of psychostimulant use by demonstrating effects on gray matter morphology
(6) . Our longitudinal approach enabled detection of correlates of psychostimulant treatment on the rate of cortical development; this was not possible in our earlier cross-sectional analysis comparing cortical thickness at study entry in groups with differing psychostimulant histories
(13) . Additionally, by using the metric of cortical thickness, determined at over 40,000 cortical points, we were able to detect more localized changes missed by lobar volumetric studies. The on- and off-psychostimulant groups were age-matched to ensure that any differences in cortical trajectories were not confounded by age effects.
In a recent study
(8), we demonstrated a delay in cortical maturation in most of the frontal (excluding the sensorimotor region) and temporal cortex using the age at peak cortical thickness as a developmental marker. Our ability to assess whether psychostimulant treatment contributes to this phenomenon is limited, because in the present study we focused on the adolescent phase of cortical thinning and did not examine the childhood phase of increase in cortical thickness. However, some considerations argue against psychostimulants being a major factor in the altered timing of maturation. The regions that were sensitive to medication were highly focal (unlike the disturbance in timing of maturation, which involved most of the cortex) and encompassed areas with both late (the dorsolateral prefrontal regions) and early maturation (the motor regions).
At the time of the first scan (around age 12) the ADHD medication groups did not differ significantly from each other in cortical thickness in the regions shown in
Figure 1, perhaps reflecting their similar history of medication exposure prior to the first scan. In prefrontal regions, the typically developing group attained peak cortical thickness earlier and thus entered the phase of cortical thinning earlier than did those with ADHD
(8) . However, the typically developing group also reached a higher peak (i.e., a thicker cortex) and thus started thinning from a higher baseline. By age 12, the ADHD and typically developing cohorts we reported on previously
(8) did not differ significantly in estimated cortical thickness in the prefrontal regions shown in
Figure 1 (top left image).
In this observational study, it is important to consider the possibility that the group differences in cortical trajectories are attributable to other dimensions on which the groups differ. The groups did not differ in initial clinical characteristics or clinical outcome, so the findings were not related to differences in the severity of the disorder or clinical course. The groups also did not differ significantly on other variables known to affect cortical trajectories, such as gender and intelligence. Of course, the ideal study design for this question would be a randomized trial comparing cortical growth in children on psychostimulants against an unmedicated comparison group—but this would be both logistically and ethically challenging. Other limitations of the present study include the lack of external validation of treatment histories, which were based purely on patient and parent report. It is impossible to exclude neuroanatomic effects of the nonpsychostimulant medication received by the ADHD groups, although the prevalence of nonpsychostimulant medication use was low and did not differ between groups at the time of final assessment.