Neurocognitive impairments in schizophrenia are well established (
1) and considered candidate endophenotypes that may facilitate understanding of genetics and pathophysiology (
2). Attention, verbal memory, and working memory domains may meet key criteria as schizophrenia endophenotypes (
3), including association with the illness, trait-like features, impairment in biological relatives, and heritability (
4,
5). However, two important aspects of endophenotype candidacy need further elucidation.
First, it is unclear whether heritability estimates of endophenotypes, and their utility in the schizophrenia gene hunt, transfer across populations. Because the genetic basis of schizophrenia in the African American population likely derives from both Africa and Europe, studies of Europeans may not yield endophenotypes that are informative for genetics in African Americans (
6). Although significant heritability of some neurocognitive domains has been reported in schizophrenia (
2,
7), it appears that no studies have examined heritability in African American families. Heritability estimates of general cognitive ability can vary according to experimental design and study assumptions (
8,
9). A meta-analysis of general cognitive ability (
8) estimates its heritability to be 48%. Results from the few studies of African Americans (e.g., reference
10) are consistent with heritable variation for general cognitive ability. However, little is known about heritability of specific neurocognitive domains, particularly in African American schizophrenia patients and their families.
Second, endophenotype criteria specify cosegregation with illness in families (
4). Thus, against a backdrop of impaired performance in relatives, individuals with schizophrenia and related disorders are more likely to exhibit the endophenotype than relatives with nonpsychotic disorders or no psychopathology. Meta-analytically derived effect sizes (
11,
12) suggest that, on average, schizophrenia patients have greater neurocognitive impairment than their biological relatives, who are more impaired than comparison participants. However, the relationship between neurocognition and nonpsychotic psychopathology in relatives is not well delineated (
11,
13). Because some neurocognitive impairments are observed in other nonpsychotic groups (e.g., mood disorders) (
14,
15), estimates of neurocognitive deficits in relatives may be affected by inclusion of relatives with psychopathology, especially if compared to psychopathology-free controls (
11). Yet inclusion of relatives with nonpsychotic illness is common in schizophrenia family studies, and there is no consensus on methods of investigating the relationship between neurocognition and psychopathology in such families (
13).
Three strategies have been employed: 1) inclusion of all relatives without evaluating relationships between neurocognition and psychopathology, 2) a priori exclusion of relatives with psychopathology, and 3) post hoc exclusion of relatives with psychopathology to determine whether differences between relatives and comparison subjects are upheld. Because other disorders may share genetic susceptibility (
16) and neurocognitive impairment (
17) with schizophrenia, a risk of strategy 1 is that relatives with psychopathology may contribute to a misinterpretation of deficits as specifically associated with schizophrenia genetic liability, thus obscuring potential overlap among disorders. Conversely, strategy 2 risks excluding genetically informative individuals. Strategy 3 strengthens conclusions regarding impaired neurocognition in psychiatrically healthy relatives but does not explicitly inform understanding of neurocognition in relatives with nonpsychotic psychopathology. A solution is to subgroup relatives according to psychopathology and directly compare across disorders, but this approach requires large study groups. The only large-scale (N=214) study of which we are aware that employed this strategy (
7) showed that family members with schizophrenia or schizoaffective disorder and those with bipolar disorders were comparably impaired on several traditional neurocognitive tasks, whereas relatives with major depressive disorder were less impaired. Although informative, that study did not investigate other forms of psychopathology in relatives, and it examined a specific population (Latino) and thus requires replication in other populations.
The Project Among African-Americans to Explore Risks for Schizophrenia (PAARTNERS) addresses the aforementioned gaps. PAARTNERS is an eight-site investigation aimed at identifying schizophrenia liability genes. The large study group (N=1,872) and standardized clinical and neurocognitive data collection provide a unique opportunity for investigating heritability and the relationship between psychopathology and neurocognition in families. PAARTNERS employed a computerized neurocognitive battery adapted for use in large-scale multisite studies, and we believe that it provides the most comprehensive neurocognitive assessment in African Americans thus far (
6).
Two related hypotheses were tested: 1) neurocognitive domains identified as candidate schizophrenia endophenotypes are heritable in African American families and 2) African American individuals with schizophrenia or schizoaffective disorder and their relatives—both affected and unaffected with other forms of psychopathology—exhibit lower neurocognitive functioning than community comparison subjects, with the greatest impairment observed in individuals with psychotic disorders.
Method
PAARTNERS methods are detailed elsewhere (
6) and will be outlined here. After complete description of the study, written informed consent was obtained. Research protocols were approved by each site's institutional review board for use of human subjects.
Participants
PAARTNERS recruited families of probands with schizophrenia, the depressed subtype of schizoaffective disorder, and the bipolar subtype of schizoaffective disorder. The probands were self-identified African Americans with at least a 6-month period (current or past) of meeting criteria for the psychotic disorder without substance abuse or dependence. Family members were selected to meet criteria for at least one of the following pedigree structures: affected sibling pair (proband and one or more siblings diagnosed with schizophrenia, schizoaffective-depressed, or schizoaffective-bipolar; N=70), trio (proband and either two parents or at least one additional sibling if a parent was unavailable; N=436), or multiplex (proband and one or more affected first-degree relatives and eight or more additional first- to fourth-degree relatives; N=28). In families with trios or affected sibling pairs, if a parent was unavailable, half-siblings were tested if they were children of the missing parent. Relatives unaffected with schizophrenia or schizoaffective disorder had to be at least 15 years old, and probands and relatives with schizophrenia or schizoaffective disorder had to be at least 18. During semistructured interviews with the 1,538 probands and relatives regarding ethnicity, 94.7% of the family members (N=1,457) identified one or more African American ancestors. The majority (3.9%, N=60) of the remaining participants had information on only 1 forbear, and no participants indicated that their ancestors were all of European descent.
Healthy community comparison subjects also self-identified as African American were group matched by geographical region to the schizophrenia patients and were targeted to include individuals in the same demographic groups (sex, age, maternal education) as the patients and relatives. They were initially identified and screened by a professional survey company employing a telephone list constructed by using zip codes in the eight geographical regions of the collaborating sites. Individuals thus identified were then contacted by the respective local sites for final eligibility determination and scheduling. To supplement this group, local recruitment of comparison subjects was also conducted at most sites through established mechanisms.
All participants were required to be proficient in English, provide informed consent, and not have mental retardation or physical conditions restricting their ability to participate in neurocognitive testing. Comparison subjects were excluded for current or past significant head injury, neurological or severe systemic illness that may affect neurocognitive functioning, current or past psychosis, another axis I disorder with less than 1 month of psychiatric stability, substance abuse in the past 1 month, substance dependence not in remission for 6 months, current or past treatment with antipsychotic agents, ECT in the past 6 months, or a history of psychosis in a first-degree family member or an unknown family history (e.g., due to adoption).
Diagnostic Assessment
DSM-IV diagnoses were assigned by using a lifetime best-estimate final diagnosis approach incorporating the Diagnostic Interview for Genetic Studies (
18), the Family Interview for Genetic Studies (
19), medical record review, and consensus review of all materials by at least two investigators. Quality assurance of diagnostic procedures was described previously (
6).
Computerized Neurocognitive Battery
The computerized neurocognitive battery, developed and validated (
20,
21) for large-scale studies, was administered by using clickable icons on desktop or laptop computers in a fixed order. The PAARTNERS battery, described in detail elsewhere (
6), uses 14 tasks to assess 10 neurocognitive domains: 1) abstraction and mental flexibility—Abstraction and Working Memory Task (
21,
22) and Penn Conditional Exclusion Test (
23); 2) attention—Penn Continuous Performance Test, Number and Letter Version (
24), Letter-N-Back, 0-back condition (
25); 3) working memory—Letter-N-Back, 1- and 2-back conditions (
25); 4) verbal memory—Penn List Learning Task, Computerized Penn Word Memory Test (
26); 5) face memory—Penn Face Memory Test (
26); 6) spatial memory—Visual Object Learning Test (
27); 7) language—Penn Verbal Reasoning Test (
20,
28); 8) spatial processing—Computerized Judgment of Line Orientation (
20); 9) sensorimotor processing—Computerized Finger-Tapping Task (
29) and Motor Praxis Test (
29); and 10) emotion processing—Penn Emotion Recognition Test (
30) and Penn Emotion Discrimination Task (
31). Administration time was approximately 120 minutes, including rest. Scoring was automated, as was downloading to a central repository at the University of Pennsylvania. For each domain except sensorimotor processing, two summary functions were calculated: accuracy (number of correct responses) and speed (response time for correct answers). For sensorimotor processing, only the speed summary function was calculated.
Statistical Approach
Heritability
For the heritability analyses, the number of individuals with measurements varied between 1,271 and 1,432. Because heritability analysis requires complete valid pedigrees, for any incomplete pedigrees, intervening but unassessed relatives were added by using pedigree information collected from participating family members. Within our pedigrees, 1,432 individuals were assessed and 765 were added to generate complete pedigrees containing 2,197 individuals. Heritability for each neurocognitive domain was estimated by mixed-model analysis of variance. The model included fixed effects for site and diagnosis, covariates for sex and age, and random effects for individuals and residual variation. Estimates for genetic and residual components of variance were determined by means of restricted maximum likelihood (
32). We applied the average information (
33) algorithm, as implemented in AIREMLF90 (http://nce.ads.uga.edu/~ignacy/newprograms.html), because it guarantees faster convergence to a solution than does the more traditional expectation-maximization algorithm (
34). Approximate standard errors for heritability estimates were determined by using the formulas of Gilmour et al. (
35). A procedure based on Fisher's z transformation (
36,
37) was used to obtain 95% confidence intervals for heritability estimates.
Group differences
Genetically related individuals are expected to show positive correlation for neurocognitive performance reflecting shared environmental and genetic variation. To account for relatedness, we used generalized estimating equation and mixed linear models with a family effect. Because the results were similar for the two types of models, only results from the generalized estimating equation models are reported. These specify the correlation structure among family members to correct for correlations among relatives. For each neurocognitive task, the correlation of siblings' scores was first estimated by fitting a generalized estimating equation model to data from only nominal full siblings. The estimated correlation from full siblings was taken as the approximation of the correlation for all relative pairs. While this correlation is expected to overestimate the correlation for other kinds of relative pairs, it provides a conservative correction for significance testing. For each function and domain, a significant type 3 generalized estimating equation analysis was followed by least squares mean difference tests to detect pairwise differences between groups. Effect sizes (d) were calculated and interpreted according to Cohen's guidelines: 0.2=small, 0.5=moderate, 0.8=large.
To evaluate the relationship between diagnostic status and neurocognitive measures, we employed two distinct encodings of diagnosis. First, we used a hierarchical, mutually exclusive encoding roughly approximating DSM-IV axis I classification. Under this traditional scheme, each individual is categorized into only one of seven diagnostic groups based on DSM-IV criteria, as in
Table 1. This scheme does not allow for comorbidity of disorders; for example, a participant with both schizophrenia and a substance-related disorder would be categorized in the schizophrenia group and the substance disorder would be effectively ignored. Thus, we also employed a dichotomous (Bernoulli) encoding in which each individual received a value of 1 (diagnosis given) or 0 (diagnosis not given) for each of 10 diagnostic categories. This dichotomous coding scheme provides for actual DSM-IV diagnostic categories that are not mutually exclusive, allowing for a fuller evaluation of the association between "secondary" disorders (such as substance-related disorders) and neurocognition. The hypothetical participant would thus receive a value of 1 for schizophrenia, 1 for substance-related disorder, and if there are no other comorbid disorders, 0 for all other categories.
Preparatory Analyses
For neurocognitive tests with skewed distributions of raw scores, log or Box-Cox transformations were performed to approximate normality. Scores were then standardized to z-scores by using the comparison subjects' mean and standard deviation. When a domain comprised multiple tests, a z-score average was computed. Because the original speed measure was time (milliseconds), we reversed the sign of the z-scores for speed to standardize the interpretation (higher value is better).
Results
For neurocognitive tests with skewed distributions of raw scores, log or Box-Cox transformations were performed to approximate normality. Scores were then standardized to z-scores by using the comparison subjects' mean and standard deviation. When a domain comprised multiple tests, a z-score average was computed. Because the original speed measure was time (milliseconds), we reversed the sign of the z-scores for speed to standardize the interpretation (higher value is better).
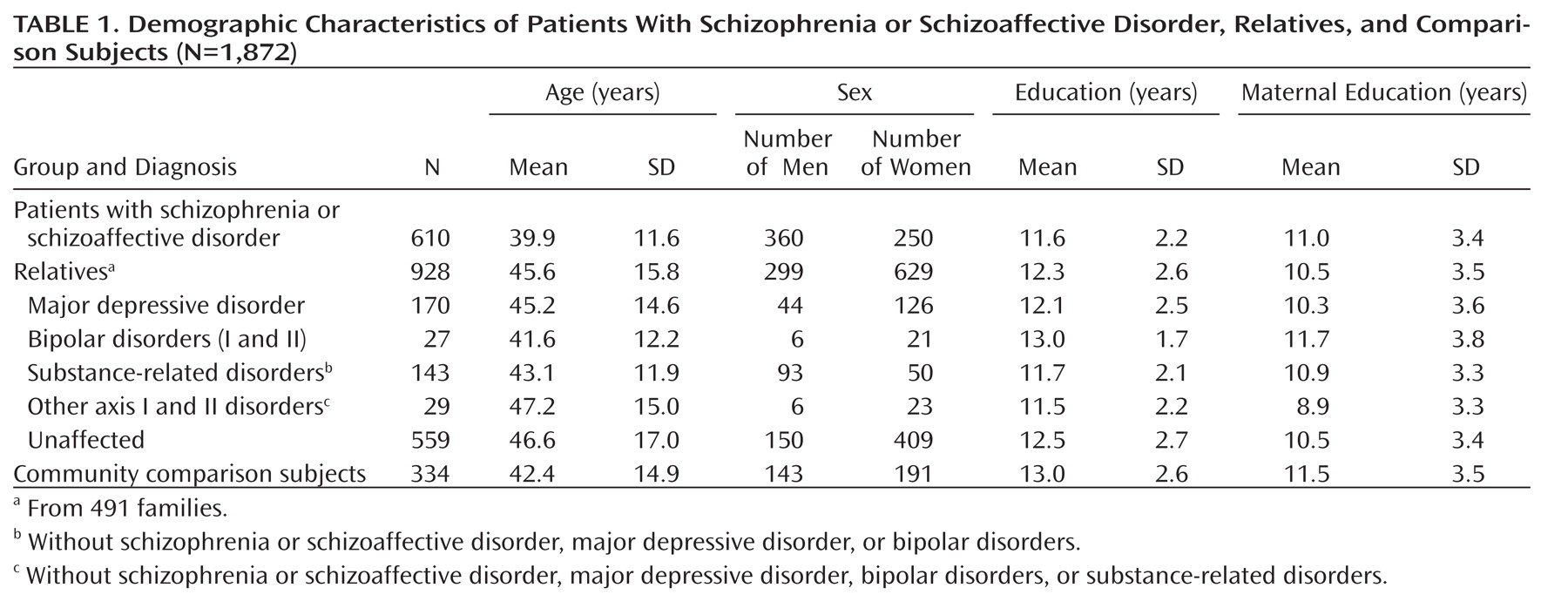
Demographic Characteristics
Table 1 presents the current PAARTNERS study group (491 families and 334 comparison subjects) with neurocognitive data. Because the participant groups differed in sex distribution and age (p<0.001), these variables were used as covariates in all models.
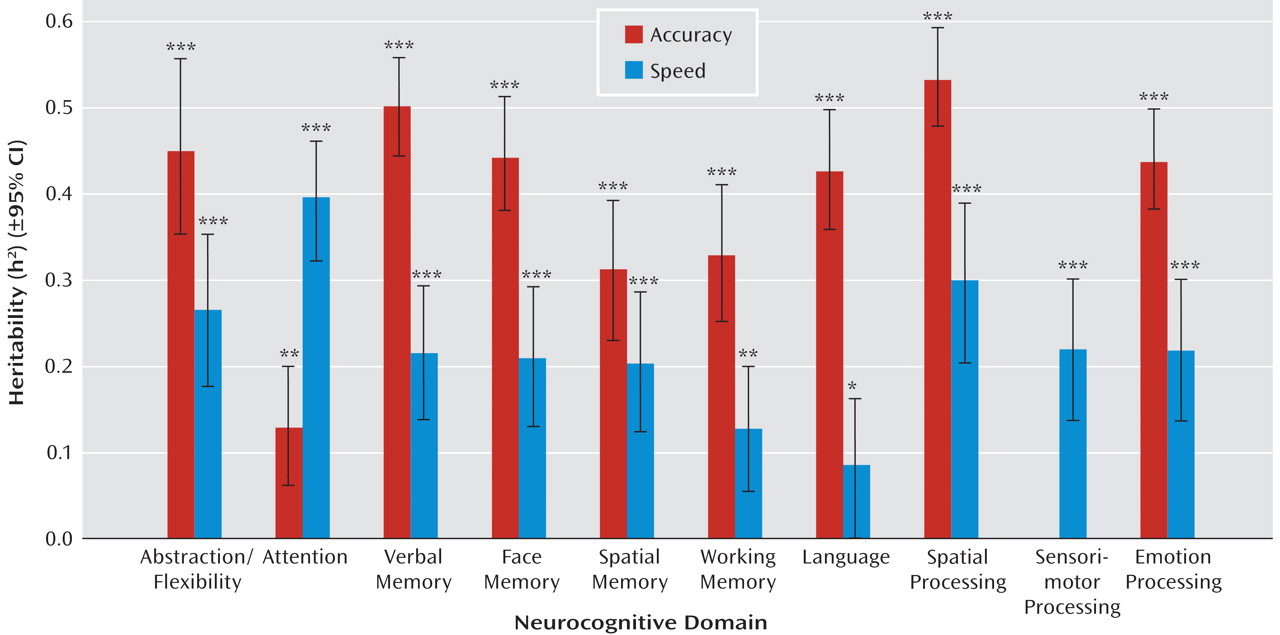
Heritability
Accuracy measures in all domains were significantly heritable; most estimates were greater than 0.30 (
Figure 1). Notably, large heritabilities were observed in domains particularly implicated in schizophrenia, including abstraction/flexibility (h
2=0.46), verbal memory (h
2=0.50), and face memory (h
2=0.44). The heritability estimates of the accuracy of spatial processing (h
2=0.53), emotion processing (h
2=0.44), and language (h
2=0.43) were also high, although these abilities have not been widely investigated as candidate endophenotypes in schizophrenia. In the domain of attention, the heritability of accuracy was low (h
2=0.13), but for speed it was high (h
2=0.40). Other than attention, heritability estimates of speed were generally lower (h
2 range=0.09–0.30) than those for accuracy (h
2 range=0.13–0.53).
Neurocognition and Hierarchical Diagnoses
Analysis of variance indicated that, with the exception of language speed, the neurocognitive domains differed among the diagnostic groups. Given the large number of variables and groups, the results are depicted in two ways.
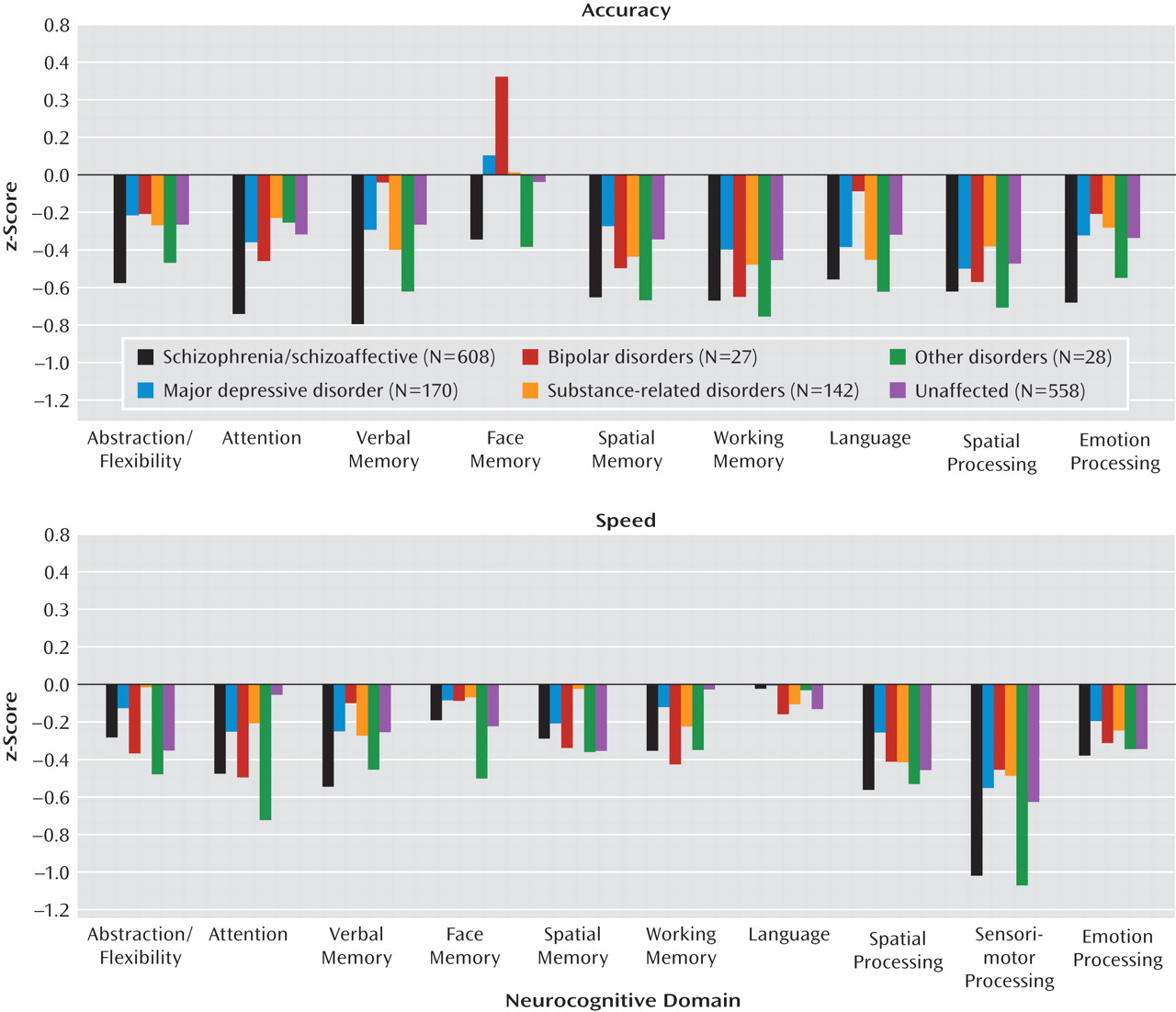
Figure 2 provides a visual representation of mean z-scores for each diagnostic group. The complementary
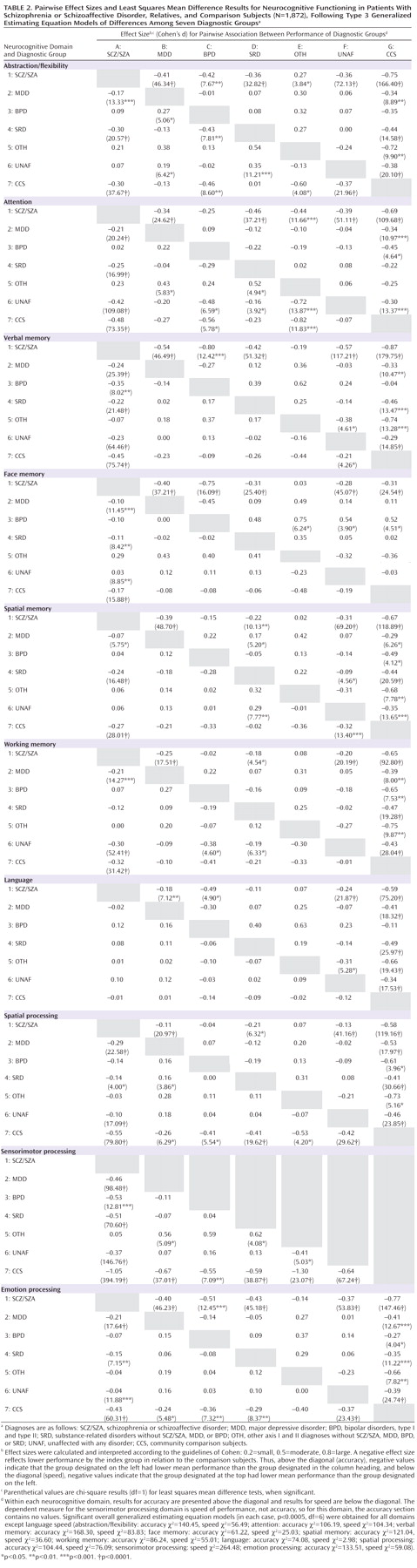
Table 2 separately gives the effect size of each pairwise group comparison for accuracy (above diagonal) and speed (below diagonal), as well as the results of significance tests comparing the group means. Entries in Table 2 are identified in this text by row and column as in a spreadsheet (e.g., row 1 in column G is referred to as 1G).
Accuracy
The z-scores for accuracy are shown in the upper part of Figure 2. Least-squares mean differences indicate that the participants with schizophrenia or schizoaffective disorder were less accurate than the community comparison subjects in all neurocognitive domains, with effect sizes ranging from small (–0.31) to large (–0.87) (Table 2, cell 1G in all domains).
The group with schizophrenia or schizoaffective disorder was also less accurate than the unaffected relatives, relatives with major depressive disorder, and those with substance-related disorders in nearly all neurocognitive domains (Table 2, 1B, 1D, and 1F in all domains). Participants with schizophrenia or schizoaffective disorder were less accurate than relatives with bipolar disorders (N=27; Table 2, 1C) in abstraction/flexibility, verbal memory, face memory, language, and emotion processing. In contrast, no significant difference was seen between schizophrenia/schizoaffective and bipolar disorder in working memory (d=–0.02) and spatial processing (d=–0.04), indicating some performance similarities in these groups. Like the bipolar group, the "other" group was quite small (N=28), but unlike the bipolar group, it differed from the schizophrenia/schizoaffective group only in attention and abstraction/flexibility (Table 2, 1E). The heterogeneous "other" group includes individuals with psychotic disorders; hence, it is expected to share some neurocognitive features with schizophrenia.
There were few significant differences in accuracy among relatives in the various diagnostic categories, reflecting similar levels of performance accuracy in relatives regardless of diagnosis (Table 2, 2C–F, 3D–F, 4E–F, 5F).
Across all diagnostic groups, relatives had significantly less accuracy than the community comparison subjects in spatial memory, working memory, spatial processing, and emotion processing (Table 2, 2G to 6G in each domain). All relatives except the bipolar group had impaired accuracy in abstraction/flexibility, verbal memory, and language. The relationship between group membership and performance accuracy was the weakest for face memory, where only relatives with bipolar disorder differed from community subjects (Table 2, 3G), and their performance was actually better than that of the community comparison subjects. An important finding was that the relatives who were unaffected with any disorder were less accurate than the comparison subjects in all domains except face memory, indicating that impairments are observable even in psychiatrically healthy relatives (Table 2, 6G).
Speed
Group comparisons for speed are displayed in the lower portion of Figure 2 and in the results below the diagonal for each domain in Table 2. Except for language, the patients with schizophrenia or schizoaffective disorder were slower than the community comparison subjects across all domains (Table 2, 7A), most markedly in sensorimotor processing speed (d=–1.05).
The patients were also slower than the relatives with major depression in all domains except language (Table 2, 2A), and they were slower than relatives with substance-related disorders in all domains except language and working memory (Table 2, 4A). In contrast, they did not differ in speed from relatives with "other" disorders (Table 2, 5A), and they differed from relatives with bipolar disorders on only verbal memory and sensorimotor dexterity (Table 2, 3A).
As with accuracy, relatives with most forms of psychopathology were comparable in response times to each other and to unaffected relatives (Table 2, 3B, 4B–C, 5B–D, 6B) with a few exceptions.
Fewer differences between relatives and the community comparison subjects were observed in speed (Table 2, 7B–F) than in accuracy. The most consistent differences across groups were found for sensorimotor speed and spatial processing (where all groups were slower than the comparison subjects) and for emotion processing (where all relative groups except "other" showed slower response times than the comparison subjects). In addition, unaffected relatives were slower than comparison subjects (Table 2, 7F) in abstraction/flexibility, verbal memory, and spatial memory, while relatives with bipolar disorder (Table 2, 7C) and "other" disorders (Table 2, 7E) were slower in abstraction/flexibility and attention.
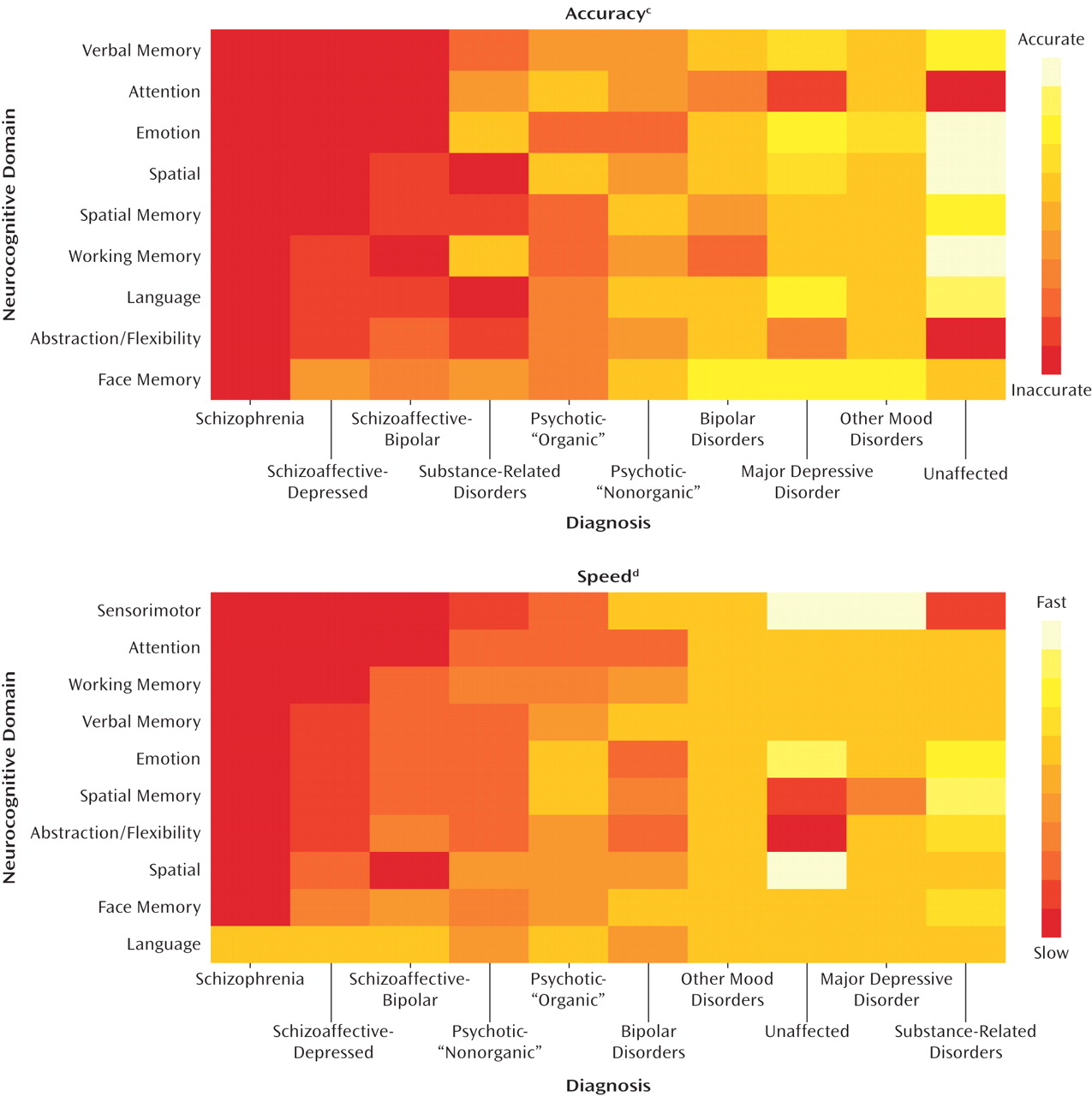
Neurocognition and Dichotomous Diagnoses
Analysis of variance related the 10 dichotomous encodings of diagnoses to the neurocognitive measures. In the form of a heat map,
Figure 3 graphically shows the relationship between neurocognitive performance and the diagnostic categories not required to be mutually exclusive. Figure 3 shows that the schizophrenia diagnosis is most significantly associated with lower accuracy on the neurocognitive measures, followed by schizoaffective-depressed, schizoaffective-bipolar, and then the remaining diagnoses. An important observation is that although substance-related disorders are associated with cognitive impairments, schizophrenia and schizoaffective disorder have stronger associations, indicating that substance disorders do not account for the neurocognitive impairments observed in schizophrenia. The dichotomous coding analyses thus reveal a direct connection between neurocognitive accuracy and schizophrenia, even after accounting for diagnostic comorbidity.
The schizophrenia and schizoaffective categories were associated with the slowest speeds, but although substance-related disorders were associated with lower accuracy, they were not as strongly associated with impaired speed.
These results underscore the relationship between schizophrenia diagnosis and neurocognitive performance, with the schizophrenia diagnosis more highly associated with reduced accuracy and speed than nonschizophrenia disorders. Relatives with schizoaffective and other disorders share many of these features, but not to the same extent. These results highlight the utility of considering multiple diagnoses per individual, rather than restricting analyses to mutually exclusive, primary diagnostic categories.
Medical History
Because PAARTNERS participants were not excluded for medical conditions that may affect neurocognition, we evaluated the relationship between neurocognitive performance and medical history. We introduced into our models three levels of medical conditions, based on relationship to neurocognition: medically healthy (N=745), minor medical condition (e.g., hypertension, minor head injury, diabetes; N=969), and major medical condition (e.g., meningitis, stroke, significant head injury, thyroid disease; N=62). The remaining individuals had an unknown medical history (missing or incomplete information, N=96) and were excluded from further medical history analyses. Generalized estimating equation analyses performed with each of the neurocognitive domain scores as the dependent variable revealed that medical history was a significant predictor of attention accuracy and the speed of verbal memory, spatial memory, spatial processing, and sensorimotor processing. However, in each case where diagnosis was a significant predictor, it remained so. Moreover, the amount of variance in neurocognitive measures that was attributable to diagnosis did not differ substantially after medical history was included in the model.
Discussion
To our knowledge, PAARTNERS has recruited and assessed the largest group of African American families of probands with schizophrenia or schizoaffective disorder to date. Heritability estimates for all neurocognitive abilities were significant, with the highest for the accuracy of spatial processing, verbal memory, abstraction/flexibility, face memory, and emotion processing and for the speed of attention (Figure 1). These results are consistent with prior work robustly implicating deficits in verbal memory, abstraction/flexibility, and attention as candidate endophenotypes of schizophrenia (
2). In contrast, spatial processing and emotion processing have not been widely regarded as candidate endophenotypes of schizophrenia. Nonetheless, both domains show high heritability and differentiate individuals with schizophrenia or schizoaffective disorder and their family members from community subjects. These findings justify further consideration of these abilities as candidate endophenotypes (
2).
Two multisite studies have supported the heritability of particular measures of the University of Pennsylvania computerized neurocognitive performance battery in families of schizophrenia probands that were predominantly European American (Consortium on the Genetics of Schizophrenia; 38, 39) or wholly European American (multigenerational investigation; 3). The results of the current investigation support the hypothesis that neurocognitive abilities, especially those implicated as candidate endophenotypes in schizophrenia, are heritable in African American families as well.
Consistent with the endophenotype criterion that the characteristic cosegregate with illness in families (
4,
5), a diagnosis of schizophrenia or schizoaffective disorder had a profound impact on neurocognitive performance, other psychotic and bipolar disorders had a more modest effect, while family members with nonpsychotic disorders only occasionally differed from unaffected family members in neurocognitive performance accuracy. Differences between schizophrenia and schizoaffective disorder were also observed (Figure 3). Schizophrenia was associated with more impaired neurocognitive accuracy and speed than schizoaffective disorder. Presently, there is no consensus on the genetic relationship between schizophrenia and schizoaffective disorder, but the literature suggests potential genetic overlap (
16). Future analyses of PAARTNERS data will seek to examine differences among family members with these disorders to gauge genetic implications.
Individuals with schizophrenia or schizoaffective disorder and their relatives with bipolar disorder had similar results for accuracy of working memory and spatial processing and for performance speed across neurocognitive domains (Figure 2), but they differed substantially on accuracy for several other domains, such as verbal and face memory. Evidence for similar neurocognitive functioning in some domains is consistent with the suggestion that schizophrenia and schizoaffective disorder share some endophenotypic markers with bipolar disorder (
14) and that they have shared genetic susceptibility (
16). This result should be interpreted cautiously because the bipolar group was small. Moreover, all relatives were genetically related to probands with schizophrenia or schizoaffective disorder, and a lifetime history of bipolar disorder in one of these family members could be etiologically or phenomenologically different from bipolar disorder in other familial contexts.
Although significantly heritable, accuracy of face memory did not differentiate relatives from community subjects, a finding inconsistent with our prior work with predominantly European American groups (
3,
40,
41). Surprisingly, the small number of relatives with bipolar disorder were significantly more accurate on face memory than community comparison subjects. A recent study using the same face processing measures supports an "other-race effect" in face processing among European Americans and African Americans (
42), but this would not seem to account for the better performance in relatives, especially because all participants in the current study were African American. Moreover, the schizophrenia patients exhibited the expected impairments. Future efforts will be aimed at examining face memory performance across relatives from racially diverse families to better understand this effect.
Relatives of patients with schizophrenia or schizoaffective disorder, regardless of axis I psychopathology, were less accurate than community comparison subjects in most neurocognitive domains, and they were slower in spatial and emotion processing and sensorimotor speed. Notably, this was true even for psychiatrically healthy relatives. In addition, analyses of group differences that included only unaffected relatives age 30 or older (119 young relatives dropped) and analyses including only unaffected relatives age 25 or older (83 young relatives dropped) did not yield substantive changes in results (data not shown), indicating that the observed impairment in relatives is not attributable to a preponderance of young relatives in premorbid or prodromal stages of schizophrenia. Moreover, other, nonpsychiatric medical conditions did not interact with psychiatric status to predict performance. These results suggest that neurocognitive performance in schizophrenia families is attributable to an individual's status as a biological relative, and thus to presumed genetic liability for schizophrenia, rather than to personal psychopathology, including substance-related disorders or other medical conditions. These results, together with the evidence for heritability and cosegregation of neurocognitive abilities with the schizophrenia diagnosis, extend support for neurocognitive abilities as candidate endophenotypes of schizophrenia in African American families.
Acknowledgments
The following research faculty and staff contributed at the eight sites: University of Alabama (central administrative site)—Roberta May; Charlie Swanson, Jr., M.D.; Laura Montgomery-Barefield, M.D.; Tolulope Aduroja, M.D.; Ryan Coleman; Rakesha Garner; Lee Prichett, R.N.; Thomas Kelley, R.N.; and Marguerite Ryan Dickson, M.S.; Duke—Joseph P. McEvoy, M.D.; and Linda Blalock, R.N.; University of Mississippi—Karen Richardson, M.S.; Morehouse School of Medicine—Deirdre Evans-Cosby, M.D.; George W. Woods, M.D.; Kendaly Meadows, R.N.; Sandra Cummings M.S.W.; Cara Stephens L.C.S.W.; and Kent Baker; Medical University of South Carolina—Shirley Hendrix, Cynthia Gilliard, Wanda Smalls-Smith, and Steven McLeod-Bryant; University of Pennsylvania—Felipe Da Silva; Alexandra Duncan-Ramos, M.S.; Jarrod Gutman; CarlaAnn Henry; Paul Hughett, Ph.D.; Farzin Irani, Ph.D.; Jennifer Jackson Greene, M.S.; Stephen J. Kanes, M.D., Ph.D.; Christian Kohler, M.D.; David Rice; Devon Seward; Steven Siegel, M.D., Ph.D.; Bruce Turetsky, M.D.; and Robert Witalec; University of Pittsburgh—Joel Wood, B.S.; Mary Miller, L.P.N.; and Frank Fleischer, M.B.A.; University of Tennessee—Kristin Beizai, M.D.; Marie Tobin, M.D.; Alyssa English, M.D.; Richard Sanders, B.S.; Shelia A. Dempsey, A.D.N.; Martha Velez, C.P.S.; Marianne Smith, B.S., M.A.; Martha Garriott, M.S., N.C.C.; Nancy Fowler; Derrick W. Allen, M.S.S.W.; Phyllis Meyer, B.S., P.A.; and Lynn Heustess, B.S.
The authors thank the study participants and research faculty and staff for their time and effort, and they thank the Western Mental Health Center and Dr. Thomas Hobbs for their support and contribution to recruitment in Ensley and Birmingham, Ala.