Learning to control behaviors that conflict with societal norms is vital for the healthy psychological development of children, which is a component of their developing self-regulatory control and their progressive ability to organize their thoughts, emotions, and behaviors in order to attain their goals
(1) . Related constructs are cognitive control
(2) and, more broadly, inhibitory control
(3) . In the present review, the term “self-regulatory control” is used to encompass these capacities as well as the ability to regulate emotional responses and to inhibit temptations or impulses for immediate gratification in the service of waiting for larger more delayed rewards
(4) . Disturbances in the maturation of these capacities likely contribute to the development of a variety of psychiatric disorders in which children have difficulty regulating their thoughts, emotions, and behaviors. These disturbances may release from regulatory control, for example, an underlying urge to move or to perform a compulsive behavior.
Substantial evidence suggests that frontostriatal circuits subserve the capacity for self-regulation in both health
(5) and illness
(6) . These circuits comprise a portion of the broader cortico-striato-thalamo-cortical loops that direct information from the cerebral cortex to the subcortex and then back again to specific regions of the cortex
(7,
8) . At least five parallel loops have been identified within frontostriatal circuits, initiating from and projecting back to the 1) supplementary motor area, 2) frontal eye fields, 3) dorsolateral prefrontal cortex, 4) lateral orbitofrontal cortex, and 5) anterior cingulate cortex
(7,
8) . The first three of these loops pass through the dorsal striatum, and the last two pass through the ventromedial striatum, including the nucleus accumbens
(8) . Findings from both animal and human studies suggest that the dorsal striatum mediates habit- or stimulus-response learning
(9), while the ventral striatum mediates reward, drive, and motivation
(10) . Although both the dorsal striatum and ventral striatum respond to rewards, the dorsal striatum appears to do so only when an action is required, consistent with its putative involvement in stimulus-response learning
(11) . The prefrontal components of these pathways have long been assigned a central role in controlling thought and behavior in accord with the pursuit of future goals
(12) .
Difficulty controlling ego-dystonic thoughts, urges, or behaviors is a common characteristic of several psychiatric disorders that arise in childhood or adolescence. Tourette’s syndrome and obsessive-compulsive disorder (OCD) are among the better studied of these disorders of self-regulation. The tics of Tourette’s syndrome are typically brief, nonpurposeful or semipurposeful behavioral fragments often enacted in response to internal or external sensory cues
(13) . Sensitivity to these cues is usually experienced as a compulsory urge that is only relieved by the performance of a tic
(13) . These urges and the preoccupation with them bear a phenomenological resemblance to the obsessional urges that typically precede compulsive behaviors. In fact, patients with Tourette’s syndrome are often affected with OCD
(14) . Extensive neuroimaging evidence suggests that the pathophysiology of both disorders involves disturbances in the frontostriatal circuits that subserve the capacity for self-regulation
(15 –
19) . Anorexia nervosa and bulimia nervosa seem to share with Tourette’s syndrome and OCD this phenomenological characteristic of disordered control over behaviors or the urges to perform them. Anorexia is defined by excessive food restriction, and bulimia is defined by frequent binge eating and a concomitant sense of loss of control. The recurrent binge eating episodes, the frequency of other accompanying impulsive behaviors
(20), and the inability to inhibit the constant preoccupation with weight and body shape suggest the presence of an impaired capacity for self-regulation in bulimia. In contrast, the stringent control over food intake in anorexia suggests the presence of excessive self-regulatory control. However, anorexia shares with bulimia the inability to inhibit weight- and body shape-related thoughts, which may alternatively suggest that a reduced rather than enhanced capacity for self-regulation is present in anorexia. As with OCD, the repetitive, ritualistic, and highly controlled behaviors in persons with anorexia may represent attempts to compensate for their inability to control obsessive thoughts and anxiety
(21) . The high rates of diagnostic crossover from anorexia to bulimia further suggest that these disorders may share a common neural substrate
(22) . Consistent with the phenomenological similarities of these eating disorders with OCD, persons with anorexia and bulimia also have higher rates of OCD and obsessive-compulsive personality features than what is present in the general population
(23) . Thus, the dysregulation of frontostriatal circuits may also be a core characteristic of anorexia and bulimia.
All of these illnesses typically begin during childhood or adolescence
(24,
25), periods during which the capacity for self-regulation develops rapidly
(5,
26) . The development of this capacity parallels and is thought to depend on the maturation of frontostriatal circuits
(27 –
29) . Thus, disturbances in the maturation of frontostriatal circuits may contribute to the shared difficulties controlling thoughts, urges, and behaviors across these seemingly disparate disorders.
Normative Development of the Capacity for Self-Regulation
Experimental paradigms that study self-regulatory processes typically require participants to inhibit a more automatic behavior in favor of a less automatic one. They are therefore regarded as experimental models for studying inhibitory control. Findings from developmental studies of self-regulatory processes show that performance on Stroop, flanker, go/no-go, and stop-signal reaction time tasks continues to improve over childhood, not reaching adult levels until at least 12 years of age
(30,
31) .
The Stroop task is one of the most commonly studied of these paradigms. Inhibiting the prepotent reading response during incongruent trials requires the mobilization of attentional resources and the resolution of cognitive conflict, with down-modulation of the more automatic reading response and up-modulation of the less automatic but more task-relevant color-naming response
(32) . Brain activity when naming the colors of incongruent stimuli is greater than when naming the colors of congruent stimuli, particularly in the anterior cingulate, prefrontal and parietal cortices, and striatum, in both adults
(33) and children
(34) .
Findings from a developmental functional magnetic resonance imaging (fMRI) study of the Stroop task among healthy participants
(27) suggested that frontostriatal circuits mature as the capacity for self-regulation improves with advancing age. The study found that activation of the inferolateral prefrontal cortex and lenticular nucleus increased with age as well as response speed and accuracy, suggesting that increasing activity of frontostriatal circuits improves behavioral control as children mature. These interpretations are consistent with findings from previous developmental imaging studies of response inhibition using the go/no-go stop-signal reaction time or anti-Saccade tasks
(26,
35,
36) .
Increasing prefrontal activation during inhibitory tasks from childhood to adulthood
(2,
27,
31) likely reflects synaptic pruning and increasing myelination in the frontal lobe
(37) . Anatomical magnetic resonance imaging (MRI) studies have tracked changes in brain volume, gray matter density, and cortical thickness longitudinally in healthy individuals
(38 –
40) . Findings suggest that the prefrontal cortices that mediate more advanced, higher-order control functions mature later than do areas that subserve more basic cognitive functions, such as sensation and movement
(38 –
40) . In addition, diffusion tensor imaging studies have shown changes in prefrontal white matter during development that presumably reflect the previously documented myelination of axons during childhood and adolescence. Ongoing myelination increases the speed of neuronal communication, thus enhancing cognitive processing with increasing age
(41) .
Normal maturational trajectories in frontostriatal systems provide a reference frame from which we can identify deviant patterns of development in individuals with Tourette’s syndrome, OCD, bulimia, and anorexia. To date, however, few studies have employed a developmental perspective to investigate the pathogenesis of these disorders, which would be best captured by longitudinal studies beginning at or before illness onset. A less definitive but also less costly way to investigate atypical neurodevelopment is to use trajectory-based analyses of cross-sectional data to compare anatomical and functional imaging findings across children and adults with and without illness. However, differences across diagnostic groups in the age trajectories of imaging measures in these studies could be confounded by systematic differences across age groups in the characteristics of the patients who are recruited into the study. For example, children and adults with a given disorder may differ in their durations of illness, rates of comorbidities, and disease subtypes
(42) . Thus, different imaging findings across children and adults with Tourette’s syndrome, for instance, may be produced by compensatory behavioral, cognitive, or emotional processes that may be more developed in adults who have had the illness longer
(15) . Finally, in the absence of imaging studies of children and adolescents who have eating disorders, we can only make crude inferences about pathogenesis based on findings from animal studies or from imaging studies of adult patients.
Self-Regulatory Disturbances in Tourette’s Syndrome
Tourette’s syndrome is a childhood-onset neuropsychiatric disorder defined by the presence of motor and vocal tics
(43) . It can be conceptualized as a disorder of impaired control of sensory urges and motor behaviors
(44) . Neuroimaging evidence from studies of children and adults with Tourette’s syndrome suggests the presence of both anatomical
(15,
16) and functional
(17,
45) abnormalities in the frontostriatal circuits that subserve self-regulatory capacities. Anatomical findings include decreased caudate volumes in children and adults
(16) and larger dorsolateral prefrontal cortices in children but not in adults who have the disorder, relative to age-matched comparison subjects. The larger dorsolateral prefrontal volumes in children have accompanied less severe tic symptoms
(15) and therefore may have represented a compensatory or adaptive process that attenuates tics, consistent with the role of the prefrontal cortex in inhibiting inappropriate impulses or behaviors.
Consistent with this interpretation of the anatomical hypertrophy of the frontal cortex, an fMRI study of tic suppression demonstrated that the suppression of tics in adults with Tourette’s syndrome produced changes in activity of frontostriatal systems. Findings from the study revealed that frontal cortices activated prominently, and the magnitude of frontal activation correlated significantly with increased activity in the caudate, which, in turn, was associated with greater decreases in activity of the putamen, globus pallidus, and thalamus. The magnitude of the activations (caudate) or deactivations (putamen, pallidum, and thalamus) during tic suppression correlated with tic severity in the month preceding the scan. Thus, a greater capacity of the cortex to modulate activity in the basal ganglia likely attenuates tic severity. Together, these anatomical and functional findings from individuals with Tourette’s syndrome suggest that when frontostriatal control systems fail, tics may be released from inhibitory control. Therefore, the nearly ubiquitous need to suppress tics in school and other social settings likely activates prefrontal cortices frequently and powerfully
(45), possibly stimulating in them an activity-dependent neuroplastic hypertrophy.
In contrast, the smaller prefrontal volumes in adults who have Tourette’s syndrome may reflect an inability to produce this activity-dependent plastic response to repeated attempts to control tics
(15) . This inability could explain the failure of Tourette’s syndrome to remit during adolescence in the minority of patients who continue to experience tics in adulthood
(46), which was true of the adults in these imaging studies. Consistent with this interpretation, fMRI studies have reported that adults, but not children, with Tourette’s syndrome rely on exaggerated activation of frontostriatal circuits in order to achieve normal performance on the Stroop task. Presumably, impaired frontally mediated inhibitory reserve confers a need for increased functional recruitment of these cortices in order to overcome enduring difficulties with self-regulation during performance of self-regulator tasks
(17) .
The prefrontal portions of cortico-striato-thalamo-cortical circuits project to specific regions of the striatum, which, in turn, project directly or indirectly to specific regions of the internal segment of the globus pallidus and the substantia nigra pars reticulata. These nuclei then project to the thalamus, which closes the loop by projecting back to the cortex
(7) . In each circuit, information flows predominantly through either a direct or an indirect pathway, both of which are modulated by dopamine
(47) . Positron emission tomography (PET) and single photon emission computed tomography (SPECT) studies suggest that dopamine D
2 receptors are excessively sensitive in Tourette’s syndrome
(48,
49) . These findings, together with reports that the administration of dopamine antagonists reduces tic severity
(50), suggest that hyperinnervation of the striatum by dopaminergic neurons may excessively stimulate the direct pathway while inhibiting the indirect pathway in individuals with Tourette’s syndrome, thereby contributing to their difficulty in regulating their tics
(44) .
Other anatomical imaging studies have identified abnormal thinning of the sensorimotor, primary motor, and premotor cortices in children with Tourette’s syndrome, with the degree of thinning proportional to the severity of their tics
(51), as well as hypoplasia of the caudate nucleus in children and adults with the disorder
(16) . Together, these findings suggest that abnormal maturation of specific pathways in portions of cortico-striato-thalamo-cortical circuits, particularly those involving sensorimotor pathways looping between the cortex and basal ganglia, may contribute to the genesis of tic behaviors. These interpretations are consistent with a report
(52) that activation of supplementary motor area, paralimbic regions (including the anterior cingulate and insular cortices), and parietal operculum accompanies the experience of the sensory cues that precede tics.
Children with Tourette’s syndrome are commonly affected with OCD
(13) . Family and twin studies indicate that these disorders are genetically related
(53) . Both tics and compulsions present as “habit-like” behaviors that have escaped regulatory control
(13) . These phenomenological similarities between tics and compulsions and their common genetic basis suggest that Tourette’s syndrome and pediatric OCD may be manifestations of the same underlying disease process, likely sharing a common neurobiological substrate.
Self-Regulatory Disturbances in OCD
OCD is characterized by recurrent intrusive thoughts, ideas, or images (obsessions) that are often accompanied by repetitive acts (compulsions)
(43) . Its age of onset is likely bimodal, with one mode at 10 to 12 years of age and the other occurring in early adulthood
(54) . The childhood-onset form most commonly occurs in the context of a personal or family history of tic disorder (tic-related OCD)
(55) .
Findings from neuropsychological studies suggest that individuals with OCD perform poorly on tasks requiring inhibitory control of behaviors and cognitions
(56,
57) . Furthermore, self-regulatory impairments on Stroop, go/no-go
(56), and oculomotor suppression tasks
(58) correlate with the severity of symptoms, suggesting that increasing degrees of functional disturbances in self-regulatory circuits may produce increasingly more severe symptoms. OCD symptoms may represent an individual’s incapacity to shift or self-regulate attentional resources away from his or her focus on obsessions
(57), as well as a failure to regulate compulsory behaviors, similar to the failure to regulate tic behaviors in persons with Tourette’s syndrome.
Anatomical findings suggest that abnormalities in frontostriatal circuits involving the orbitofrontal cortex, anterior cingulate cortex, and striatum may contribute to the development of OCD
(18,
19,
59) . However, the presence of undiagnosed comorbid tic disorders could also account for striatal abnormalities in children with OCD and in adults with childhood-onset OCD
(18,
60) . Findings of increased anterior cingulate volumes in children
(18,
61) contrast with findings of normal volumes in adults with OCD
(19,
59) . Reports of increased gray matter in the orbitofrontal cortex of children with OCD
(60) also contrast with findings of reduced volume of the orbitofrontal cortex in adults
(19,
62) . Longitudinal studies of child- and adult-onset OCD are needed to understand the developmental trajectories that produce these divergent morphological findings in frontostriatal pathways in studies of children and adults with OCD.
Increased activity within frontostriatal circuits during symptom provocation suggests that hyperactivity in frontostriatal circuits generates the symptoms of OCD
(63) . Alternatively, hyperactivation could represent attempts on the part of the person with OCD to inhibit the performance of compulsions or to suppress behavioral withdrawal when presented with the provoking stimulus during the scanning process
(64) . Greater activation of orbitofrontal cortices during symptom provocation in adults with OCD was in fact associated with a smaller increase in reported symptoms
(65), suggesting that activation of orbitofrontal cortices may have inhibited rather than caused symptoms. Furthermore, inverse correlations of symptom severity with activity in the right orbitofrontal and dorsal anterior cingulate cortices during response inhibition on a go/no-go task suggested that the patients who activated these regions most were better at suppressing their unwanted thoughts and compulsions
(66) . Nevertheless, findings from a wide range of studies suggest that disturbances in orbitofrontal cortex may play a causal role in producing the symptoms of OCD
(67) . These findings include anatomical abnormalities in orbitofrontal cortices of adults with OCD, the development of OCD following various naturalistic lesions of the orbitofrontal cortex, and the improvement in symptoms following neurosurgical lesions of white matter tracts leading to and from the orbitofrontal cortex
(68) .
OCD may also involve abnormalities of the action monitoring or error detection system that is mediated by the anterior cingulate
(69,
70) . Event-related potential and fMRI studies have shown increased anterior cingulate activation in adults with OCD relative to healthy comparison subjects following errors or high-conflict trials on inhibitory control tasks
(69 –
71) . Furthermore, the degree of hyperactivation is proportional to symptom severity
(69,
70) . One event-related potential study reported increased error-related anterior cingulate activity in children with OCD relative to healthy children, although the degree of activation did not correlate with symptom severity
(72) . Children with OCD continued to show error-related hyperactivity of the anterior cingulate after successful treatment with cognitive behavior therapy, suggesting that higher activity in the anterior cingulate following errors may be a trait-like disturbance in OCD. In contrast, an fMRI study reported less error-related anterior cingulate activation in adolescents with partially remitted OCD relative to healthy comparison subjects
(73) . Most of the adolescents in this small sample were taking selective serotonin reuptake inhibitors (SSRIs), which may have blunted their error-related activity. Interpreting the relevance of error-related activity of the anterior cingulate cortex to the pathogenesis of OCD is difficult because this activity may simply reflect excessive worries about or exaggerated reactions to erring on the task. Conflict-related activation of the anterior cingulate cortex following correct responses
(71) could similarly represent exaggerated arousal or even greater anxiety relative to comparison subjects while performing challenging tasks in an experimental setting.
Evidence suggests that the frontostriatal circuits that include the orbitofrontal and anterior cingulate cortices are functionally abnormal in adults with OCD and likely contribute to their symptoms of impaired regulation. The functioning of these circuits has not been investigated longitudinally in patients with OCD, however, and only two fMRI studies to date have assessed the functioning of these circuits in younger patients
(73,
74), thereby precluding our understanding of the developmental origins of frontostriatal disturbances in OCD. Whereas the pathogenesis of Tourette’s syndrome involves frontostriatal circuits consisting of motor and premotor cortices and the dorsal striatum, OCD seems to involve disturbances in the orbitofrontal and anterior cingulate portions of frontostriatal circuits. These circuit-level distinctions of frontostriatal dysfunction may contribute to the phenotypic differences between these often co-occurring conditions. In other words, the differentially segregated cortical inputs and outputs of cortico-striato-thalamo-cortical circuits may provide a neuroanatomical basis for the differential release of either tics or compulsions from regulatory control in persons with Tourette’s syndrome or OCD, respectively
(75) .
Self-Regulatory Disturbances in Bulimia Nervosa
Bulimia nervosa is characterized by recurrent episodes of binge eating (the consumption of an excessive amount of food in a short period of time) that are followed by self-induced vomiting or other compensatory behaviors that avoid weight gain
(43) . Episodes of binge eating are associated with a severe sense of loss of control
(76) . Dysfunctional self-regulation may therefore contribute to binge eating by releasing feeding behaviors from regulatory control. Dysfunction in regulatory systems may also produce an inability of persons with bulimia to control their preoccupation with body shape and weight, thereby inducing purging behaviors. Mood instability and impulsive, aggressive, and compulsory behaviors are common in individuals with bulimia, perhaps indicating the presence of more pervasive difficulties in self-regulation
(76) .
Although fMRI studies of persons with bulimia are few, the available evidence suggests that dysfunctional frontal control systems may contribute to their binge eating and impulsivity
(77) . Binge eating behaviors in persons with bulimia, similar to the defining behaviors of Tourette’s syndrome and OCD, may arise from or may at least be facilitated by the presence of dysfunctional control systems that release from inhibitory control a preexisting vulnerability to developing this particular illness. In bulimia, this vulnerability may stem from altered functioning of serotonergic systems that can produce both impulsivity and decreased satiety
(78) . Feelings of hunger in turn produce urges to binge, which may be released inappropriately by dysfunctional control systems to produce binge eating episodes. Interactions with the aesthetic cultural ideals of thinness and physical fitness then likely produce purging behaviors to counteract the weight gain that binge eating would otherwise produce.
Disturbances of serotonin metabolism within frontal cortices
(79,
80) may predispose to the development of bulimia by reducing feelings of satiety
(79) . Consistent with this possibility, treatment with SSRIs tends to decrease the frequency of binges
(81) . Moreover, serotonergic disturbances have been documented in both ill
(82) and recovered
(83) individuals with bulimia, suggesting that these may be trait-like disturbances in this population. Serotonergic abnormalities may contribute not only to feeding disturbances
(84), but also to the high levels of impulsivity reported in persons with bulimia
(85), consistent with a vast literature linking serotonin abnormalities with impulsivity in both humans and animals
(86) .
Our fMRI findings among women with bulimia suggest that their failure to engage frontostriatal circuits appropriately contributes to their impairments in behavioral self-regulation
(87) . Patients with bulimia responded impulsively and made more errors relative to comparison subjects on the Simon task, a nonverbal analogue of the Stroop task. Even when responding correctly on incongruent trials, patients failed to generate the same activity as comparison subjects in the frontostriatal pathways thought to subserve inhibitory control, including the inferolateral prefrontal and anterior cingulate cortices, inferior frontal gyrus, putamen, and caudate. In addition, those with the most severe bulimia symptoms engaged these regions the least and demonstrated the poorest performance on the task. Analyses of brain activity during the commission of errors indicated greater activation of the dorsal anterior cingulate cortex of patients during incorrect responses relative to correct responses on conflict trials, suggesting the presence of heightened error detection or performance monitoring activity. However, activation of this region did not modulate the impulsive performance of the patients on subsequent trials, since their reaction times did not slow following errors. These findings suggest that the inability of persons with bulimia to engage frontostriatal systems likely contributes to their binge eating and other impulsive behaviors.
Binge eating could also be caused by disturbances in the frontostriatal circuits that subserve motivation and reward, including the orbitofrontal cortex and ventral striatum that are involved in processing the hedonic value of food
(88) . The consumption of food is associated with increased dopamine release in these reward circuits
(89), and increases in dopamine are associated with more motivated (food seeking) behavior in rats
(90) and humans
(91) . Consistent with our model of self-regulatory disturbances in bulimia, binge eating may reflect an inability to control the temptation for immediate rewards (food consumption) in favor of a more delayed reward (a slimmer body)
(92) . Although fMRI studies have suggested that anorexia may involve dysfunction in reward circuits
(93,
94), reward processing has not yet been assessed systematically in individuals with bulimia.
Self-Regulatory Disturbances in Anorexia Nervosa
Anorexia nervosa and bulimia nervosa have similar phenotypes and clinical courses
(25) . Both primarily affect women, and both typically begin in adolescence. Anorexia includes a relentless preoccupation with body shape and weight and repetitive ritualized behaviors that maintain abnormally low body weight
(43) . Individuals with anorexia have perfectionist and obsessive temperaments that persist following recovery
(95) . Their rigid control over feeding behaviors seems to suggest the presence of pathological self-regulation.
Approximately one-third of adults with bulimia describe past histories of anorexia
(96), and a significant proportion of patients with anorexia regularly binge eat or purge
(25) . The high rates of diagnostic crossover between these disorders
(22) suggest that anorexia and bulimia, similar to Tourette’s syndrome and OCD, may be manifestations of the same or comparable underlying disease processes. In addition, their phenotypic similarities, such as an intense preoccupation with food and a high prevalence of OCD and obsessive-compulsive personality traits
(97 –
99) that predict poor outcome
(100), suggest that these disorders share neural substrates. Anorexia and bulimia therefore appear to lie on a spectrum of self-regulatory control over feeding behaviors, with excessive control present in anorexia restricting subtype, less control present in anorexia binge/purge subtype, and a paucity of control in bulimia
(101) . However, individuals with anorexia have difficulty controlling their obsessive preoccupation with thinness and their ritualistic behaviors that pertain to eating and exercising
(102) . Thus, similar to OCD, symptoms of anorexia may represent a failure to shift or to regulate attentional resources away from the intrusive thoughts that motivate calorie restriction and produce ritualistic behaviors. Alternatively, the ritualistic behaviors in anorexia and OCD may represent a failure to regulate the behaviors themselves.
Several lines of evidence point to abnormalities of the anterior cingulate cortex in both ill and recovered persons with anorexia. First, decreased volumes of the dorsal anterior cingulate cortex have been reported in ill
(103) and recovered
(104) adult patients relative to comparison subjects. Second, SPECT studies suggest that adults with anorexia have lower resting regional cerebral blood flow in the anterior cingulate cortex relative to comparison subjects, both before and after weight restoration
(105) . Third, PET studies report alterations in postsynaptic serotonin 5-HT
1A and 5-HT
2A receptors in the anterior cingulate cortex in both ill
(106) and recovered
(107) patients. These receptors mediate, respectively, the inhibitory and excitatory actions of 5-HT on cortical neurons
(108) ; thus these alterations may produce further functional abnormalities in the anterior cingulate cortex. Given the central role of this region in self-regulatory control
(27), these abnormalities seem likely to contribute to self-regulatory disturbances in patients with anorexia
(78) .
Although anorexia typically arises during adolescence
(25), imaging data from adolescent patients are sparse, possibly because the confounding effects of malnutrition make findings from studies of patients in the underweight state difficult to interpret. Reduced volumes of total gray matter have been reported in both adolescents and adults with anorexia during acute illness
(109,
110) . One study
(109) reported that these deficits persisted in the recovered state, but several additional studies
(110 –
112) have reported that gray matter volumes normalize following recovery. Whether or not these anatomical abnormalities simply reflect the transitory effects of malnourishment is unknown.
Most fMRI studies of individuals with anorexia have used symptom provocation designs that expose participants to food-related stimuli
(113,
114) . In one study
(114), for example, both ill and recovered women with anorexia activated the medial prefrontal and anterior cingulate cortices more than comparison subjects in response to food compared with nonfood stimuli, suggesting that activation of the frontal cortex to food stimuli represents trait rather than state markers in anorexia. Recovered patients engaged the lateral prefrontal cortex more than ill patients. Similar to findings from persons with OCD
(65), activation of the lateral prefrontal cortex during symptom provocation may reflect the better ability of recovered patients to control their anxiety and preoccupation with food stimuli, consistent with the role that the lateral prefrontal cortex plays in cognitive control
(12) .
Findings from fMRI studies suggest that reward processing is dysfunctional in persons with anorexia. In one study that involved a guessing game
(93), women with anorexia activated the ventral striatum similarly during their responses to both positive and negative feedback. Healthy participants, in contrast, activated the ventral striatum preferentially in response to positive feedback, consistent with prior findings of ventral striatal responses to reward but not punishment during this task in healthy participants
(115) . PET studies have also reported increased D
2 /D
3 receptor binding in the ventral striatum both before and after recovery in patients with anorexia
(94), possibly explaining their abnormal brain activity during reward processing. Dysfunction in the ventral striatum and related portions of frontostriatal circuits that subserve reward processing may contribute to a diminished motivation to eat in individuals with anorexia. Food may not be as rewarding to them as it is to others
(116) .
Dysfunction in this circuit, including abnormal activation of the ventral striatum during negative feedback
(93), may also contribute to the diminished motivation of these individuals to avoid negative consequences, such as starvation and repeated hospitalization. Whereas decreases in the numbers of striatal D
2 receptors in obese individuals
(117) may reduce self-regulatory control and promote overeating, increases in the numbers of D
2 receptors in persons with anorexia may enhance self-regulatory control and promote abstention from eating. Excessive control may thereby contribute to the remarkable capacity of individuals with anorexia to deny themselves the gratification of food ingestion in the service of attaining a thinner body.
Unrealistically high personal standards and excessive concerns with making mistakes are common in patients with either anorexia or OCD
(118,
119) . Consistent with these phenotypic traits, persons with OCD activated the anterior cingulate cortex more during the commission of errors than healthy comparison subjects on tasks that required inhibitory control, suggesting the presence of a hyperactive error monitoring system
(69,
70,
120) . However, acutely ill patients with anorexia activated the anterior cingulate cortex less than comparison subjects during the commission of errors on a flanker task
(121) . These differences in activity within error monitoring circuits across anorexia and OCD groups are surprising, given the perfectionism and excessive concern with making mistakes that are common in both disorders.
Conclusion
Substantial evidence suggests that the development of a capacity for self-regulation relies on the maturation of frontostriatal circuits. The clinical presentations of Tourette’s syndrome, OCD, bulimia, and anorexia suggest the presence of impaired control processes in each of these conditions, and converging lines of evidence suggest that the pathogenesis of each of these disorders involves anatomical and functional disturbances in frontostriatal circuits. Improving our knowledge of the maturation of self-regulatory processes and of the frontostriatal circuits that subserve them will allow us to determine how these systems can be derailed during development and how such disruptions may contribute to the development of these and other childhood disorders.
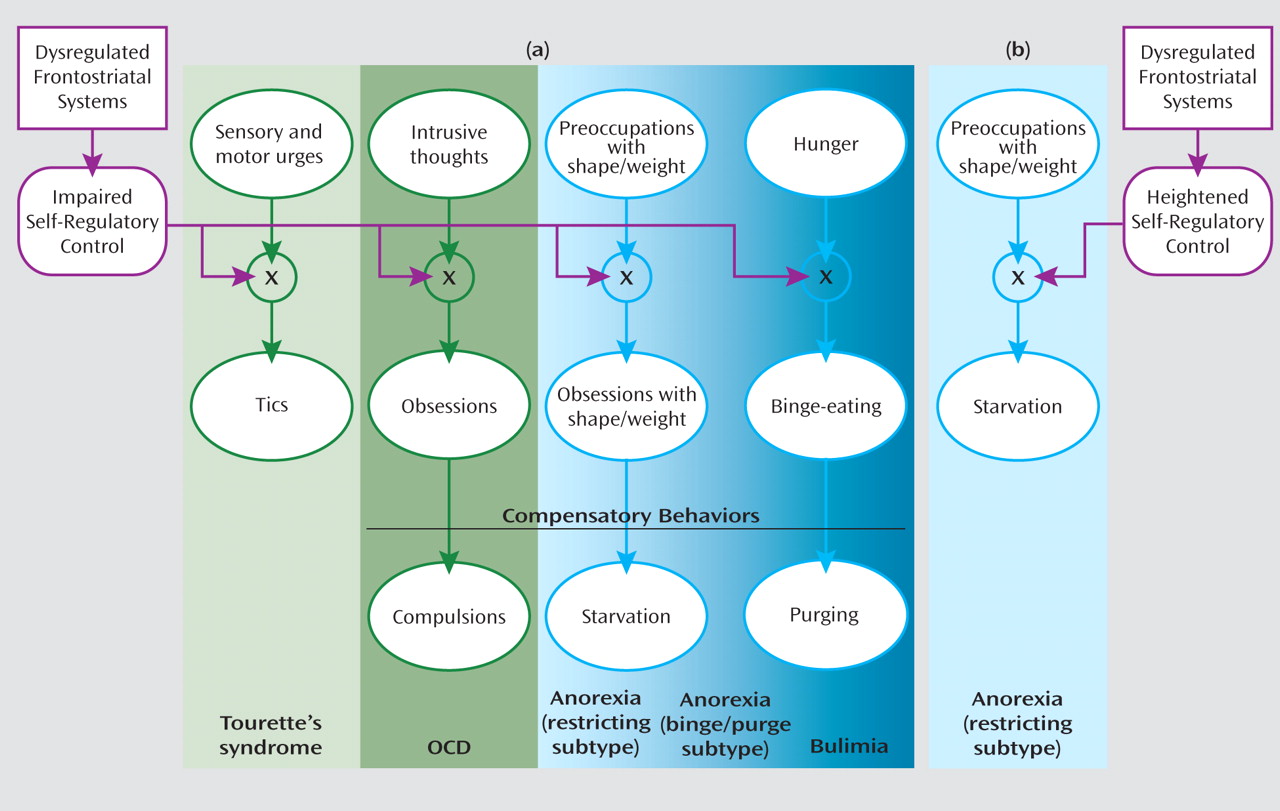
Sensory and motor urges, intrusive thoughts, hunger, and concern with body shape and weight are common in both healthy and ill individuals. Although a capacity for self-regulation keeps these urges, thoughts, and drives under control in healthy individuals, anatomical and functional disturbances of frontostriatal systems may release them from inhibitory control to allow expression of the tics in Tourette’s syndrome, the obsessions in OCD, the preoccupation with body shape and weight in anorexia, or the binge eating in bulimia (
Figure 1 ). The obsessions in OCD, the obsessive preoccupation with body shape and weight in anorexia, and the fear of gaining weight from binge eating in bulimia are predominantly ego dystonic and therefore elicit compensatory behaviors that aim to alleviate the associated anxiety or discomfort, producing compulsions, starvation, or purging, respectively. Starvation in anorexia restricting subtype may alternatively arise directly from a heightened and excessive capacity for self-regulation (
Figure 1 ).
A crucial question raised by this model is why individuals who have an impaired capacity for self-regulation do not typically develop the symptoms of all these disorders. Tourette’s syndrome and OCD likely arise from disturbances in differing portions of frontostriatal circuits. Whether anorexia and bulimia can be similarly distinguished by the involvement of different portions of frontostriatal circuits should be the focus of future research. However, anorexia and bulimia are also at least partly caused by cultural ideals of thinness
(122), whereas the clinical presentations of Tourette’s syndrome and OCD are relatively uniform in prevalence and presentation across cultures
(123), suggesting that they may have stronger biological determinants than anorexia and bulimia. Finally, differing predispositions to experience sensorimotor urges and preoccupation with food likely interact with differing capacities for self-regulation to determine who develops Tourette’s syndrome, OCD, anorexia, or bulimia and to what degree of clinical severity.
A current controversy in nosology is whether mental disorders, particularly developmental psychopathologies, are best characterized using a categorical or dimensional approach
(124) . The shared phenotypic characteristics that self-regulatory disturbances produce and the shared involvement of frontostriatal circuits across these varied disorders support, to some extent, a dimensional conceptualization of these predispositions, capacities, and manifest illnesses. However, if differing portions of frontostriatal circuits are indeed responsible for the development of one rather than another of these disorders, then categorical distinctions among them may still be most appropriate. Additional research on the neural bases of these disorders may help provide a biological resolution to this important nosological debate
(125) .