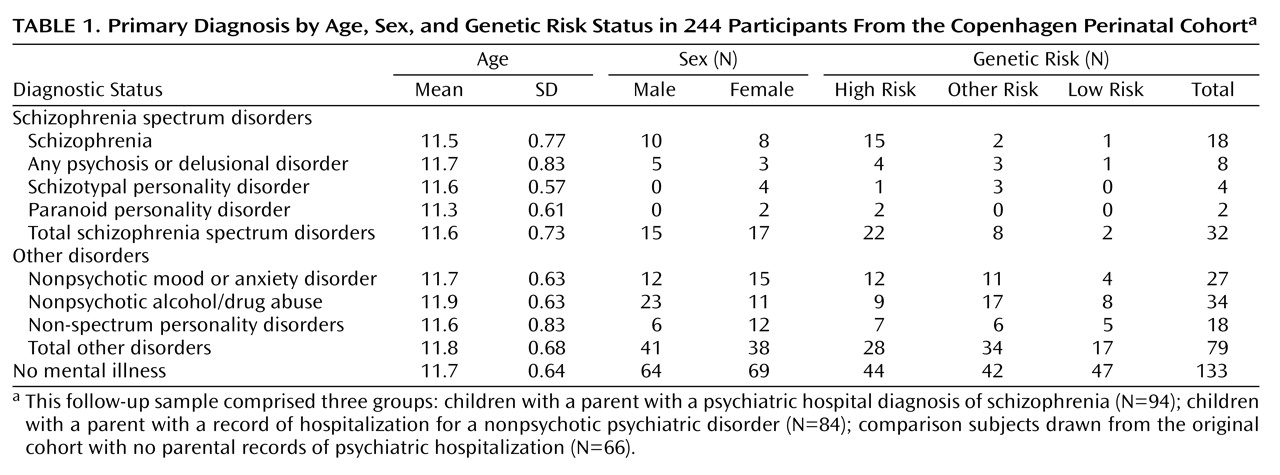
Coordination Scale
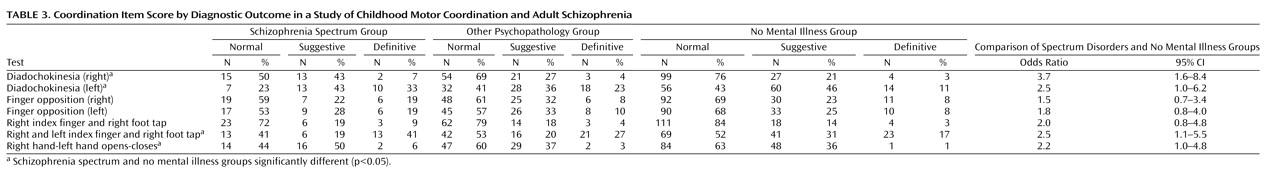
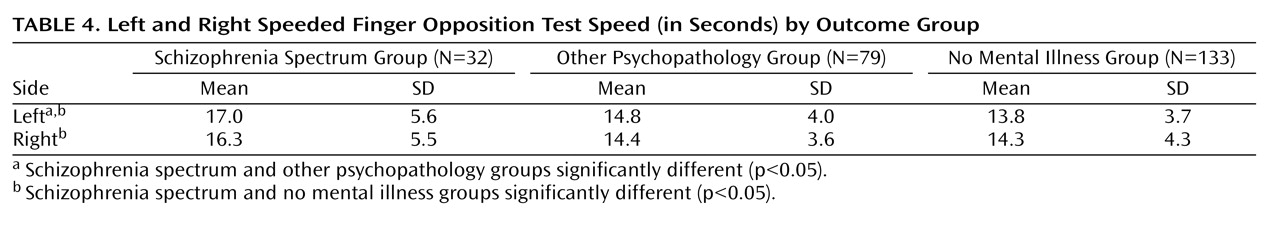
Results from this study suggest childhood differences in coordination between those who do and do not develop a schizophrenia spectrum disorder in adulthood. Coordination deficits appeared specific to the spectrum group, as participants who eventually developed a schizophrenia spectrum disorder exhibited significantly poorer premorbid coordination scores compared to those who did not develop a mental illness, and nearly significantly (p=0.08) poorer premorbid coordination than those who developed a nonpsychotic mental illness in adulthood. These results were found while incorporating genetic risk, and they held when controlling for demographic variables. Our primary analyses involved a coordination scale consisting of several individual tests of coordination. The aggregate scale provided increased statistical power to detect differences relative to a single item and yielded an effect size in the high range (Cohen’s d=0.62) when comparing the spectrum disorders group to the no mental illness group.
The finding of elevated coordination deficits among those who eventually developed schizophrenia spectrum disorders is consistent with the overall findings in this research domain as well as with the few existing prospective studies. As in the reports of the Israeli High-Risk Survey
(36), the Swedish High-Risk Survey
(9), and the New York High-Risk Project
(10), motor abnormalities detected in infancy and/or childhood were associated with an increased risk of subsequent schizophrenia spectrum disorders. Findings from this current study diverge, however, from a landmark study by Walker et al.
(17) in that the differences in motor functioning observed among infants in their study group were not observable beyond age 2. Substantial methodological differences may account for this inconsistency. Walker et al. rated motor function on the basis of the coding of spontaneous behaviors visible on home movies. In contrast, assessment of infant and child coordination in our study involved formal, highly structured, hands-on examinations performed by a pediatric neurologist. We believe that this approach is more likely to detect neurological soft signs.
This study included several other methodological advantages over previous studies, including an assessment of coordination blind to risk group and eventual diagnostic outcome; an analysis of a composite scale as well as of individual items; a familial psychiatric risk comparison group; and a relatively high number of individuals with a schizophrenia spectrum disorder at follow-up through middle age (ages 46–48). The unique methodological advantages we incorporated in this long-term longitudinal prospective study, along with previous studies documenting movement abnormalities beyond infancy
(6,
15), together increase confidence in the conclusion that coordination deficits frequently antedate the diagnosis of schizophrenia spectrum disorders, perhaps by as much as two decades, and are detectable at a variety of developmental stages.
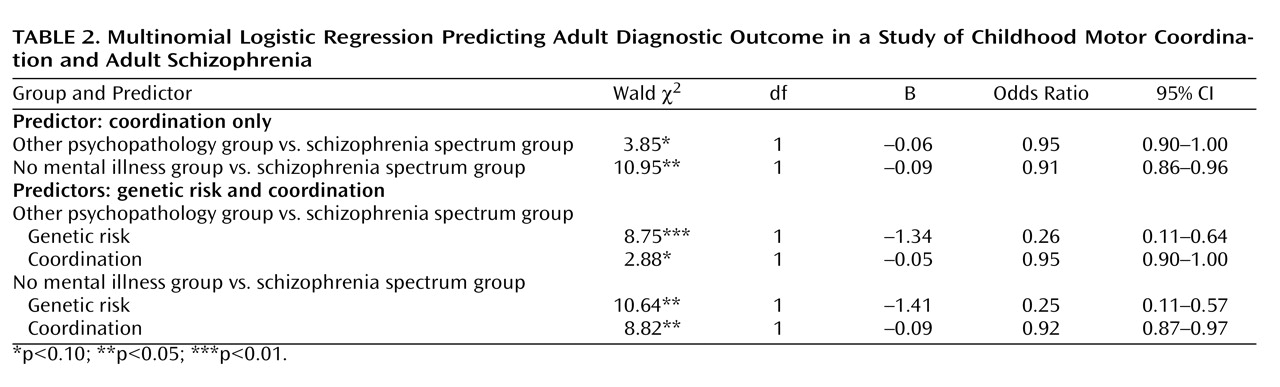
The results of this study clearly suggest direct effects between outcome and coordination and between outcome and genetic risk. The analyses did not support an interaction between genetic risk and coordination or a model whereby coordination deficits are mediated by genetic risk. Although it is important not to overinterpret null findings (especially in light of our imperfect measure of genetic risk), these findings suggest that coordination predicts over and above, and is independent of, genetic risk for schizophrenia. Possible neural and environmental explanations as to how coordination deficits might relay to adult schizophrenia outcome are described below.
Possible Mechanisms
Multiple motor systems, including the corticospinal/pyramidal, supplemental motor, basal ganglionic/extrapyramidal, and cerebellar systems, and their associated networks likely contribute to motor task performance in our coordination battery
(37,
38) . That being said, motor incoordination is classically attributed to dysfunction of the cerebellum and/or basal ganglia. This neurological understanding, originally derived from clinical-pathological correlations, has been confirmed by functional MRI studies and other strategies (see, for example, references
5,
7,
39,
40) . Therefore, we consider it likely that dysfunctions in the cerebellum, basal ganglia, or both play a role in the observed coordination deficits in those who developed a schizophrenia spectrum disorder.
Rather than abnormalities in specific brain structures such as the cerebellum or the basal ganglia, several authors argue that disruptions of specific pathways (frontocerebellar dysfunction, striatal pathology) are responsible for neuromotor dysfunction in schizophrenia
(19,
41) . For example, Mittal et al.
(40) suggest that, similar to minor physical anomalies, movement dysfunction potentially reflects subcortical brain dysfunction resulting from prenatal insults. This conclusion appears compatible with our own results, documenting coordination deficits well before symptom onset. Thus, it seems reasonable that both positions are true. That is, individuals with schizophrenia may exhibit both intrinsic dysfunction and neuroanatomical atypicality of the cerebellum and/or basal ganglia and disruption of the patterns of connectivity through which the cerebellum and perhaps the basal ganglia exert modifying effects on motor output. Regardless of the precise mechanisms and timing, the findings from this study implicate neural substrate involvement (structural, pathways, or both) in schizophrenia, early in the course of illness, prior to the emergence of psychotic symptoms.
Given the likely role of the cerebellum and other related circuits in coordination deficits, our findings might be viewed in the context of Andreasen and colleagues’ unitary model of “dysmetria”
(42) . Andreasen et al. suggest that dysfunction in the cerebellum and cortico-cerebellar-thalamo-cortical circuits might be a unifying explanation for diverse motor, cognitive, and psychiatric symptoms of the illness. Recent studies suggest that cerebellar dysfunction might underlie some of the core features of the disease (e.g., cognitive abnormalities)
(43), and our findings provide further evidence that such cerebellar dysfunction precedes illness onset. Andreasen et al. suggest that the presence of coordination deficits indicates underlying abnormalities in basic cognitive processes (e.g., perception, associations) that could lead to misinterpretation of external and internal stimuli. Misinterpretation might account for schizophrenia symptoms, ultimately taking the form of psychotic processes (e.g., hallucinations, delusions, thought disorder, and negative symptoms).
Previous studies from this project have reported other neurodevelopmental markers and precursors to schizophrenia spectrum disorders that provide additional information about regions of possible disruption as well as timing of possible insults (e.g., increased minor physical anomalies, ocular disalignment, and atypical laterality)
(22,
31,
32) . These findings, together with those from this study, support the presence of dysfunction of the cortico-cerebellar-thalamo-cortical circuit and perhaps other neural networks and processes (e.g., hemispheric asymmetry) early in life (possibly originating in the first and second trimesters of gestation), well before the onset of more downstream hallmark symptoms (i.e., delusions and hallucinations). However, additional studies are needed to pinpoint the specific regions or pathways, as well as the timing of disruption and developmental processes, that are responsible for the diverse symptomatic manifestations of schizophrenia, using more advanced techniques over time
(43) .
From a diathesis-stress perspective (emphasizing environmental stress), research suggests that poor coordination is associated with a number of social, academic, and emotional consequences
(44) . Additionally, there is evidence to support detrimental effects of coordination abnormalities over time
(45) . Beyond the neurodevelopmental implications of our findings discussed above, it is reasonable to speculate that poor coordination in childhood engenders at least some taxing psychosocial encounters that may contribute to stress within a diathesis-stress framework. It is also reasonable to speculate that these stressful events further exacerbate preexisting coordination deficits as well as neurological vulnerabilities, resulting in a self-sustaining, iterative, and perhaps progressively detrimental process between coordination and stress.
This study suffers from some notable limitations. Despite a relatively large number of individuals who developed a schizophrenia spectrum disorder, the total number of people in this group limited the statistical power for some analyses. This is particularly true of the number of individuals who developed a spectrum disorder who were not in the high-risk group, and it might also have contributed to the finding of only a trend-level difference between the schizophrenia spectrum disorders group and other psychopathology group. Another concern, shared by all research on high-risk groups, is the issue of generalizability to those individuals who develop a spectrum disorder but who do not have a parent with schizophrenia. It is likely, however, that genetic influences play a role in most cases of schizophrenia, even if the parents fail to manifest the disorder phenotypically
(46) . Finally, only one neurologist performed the neurological examinations, preventing an evaluation of interrater reliability. The neurologist was, however, highly trained and was functioning under strict research procedures and conditions.
Despite these limitations, given the strengths and uniqueness of this study, the findings advance the understanding of the development of schizophrenia in several ways. Detecting coordination deficits prospectively adds considerable support to the notion that coordination dysfunction precedes schizophrenia and may be a meaningful expression of an underlying biomarker. Applying what is known about the mechanisms of coordination deficits to the etiology of schizophrenia offers possible clues to how early neural deficits mediate the development of the disorder.