Subject Disposition
A total of 2,804 subjects were screened by phone (gate A). Of these, 1,088 signed consent for evaluation of inclusion and exclusion criteria (gate B) and 439 completed the baseline assessment and were subsequently randomly assigned to treatment (gate C). This report concerns only the 327 subjects randomly assigned to active treatment conditions: combined treatment (N=107), fluoxetine (N=109), and CBT (N=111). Of these 327 subjects, 234 (71.6%) remained in the study at week 36. Of these, 178 (54.4%) completed stage III in the treatment condition to which they had been randomly assigned. Sixty-six percent of the eligible subjects (N=215) participated in at least one stage IV assessment. During stage IV, 49 (45.8%) of the 107 in combined treatment, 46 (42.2%) of the 109 receiving fluoxetine, and 53 (47.7%) of the 111 in the CBT group completed all four stage IV assessments. The proportions were somewhat higher for those who completed an assessment at month 12: 65 (60.7%) of the 107 in combined treatment, 56 (51.4%) of the 109 receiving fluoxetine, and 67 (60.4%) of the 111 CBT subjects. During stage IV, 215 (65.7%) of the 327 families provided information on mental health services. Data collapsed across stage IV indicated that 2.8% of the subjects received inpatient mental health care, 34.0% received outpatient mental health care, 34.0% received services from a nonphysician mental health provider, and 17.2% received services from a physician mental health provider. There were no differences between treatment groups in the type of services received in stage IV.
Benefits
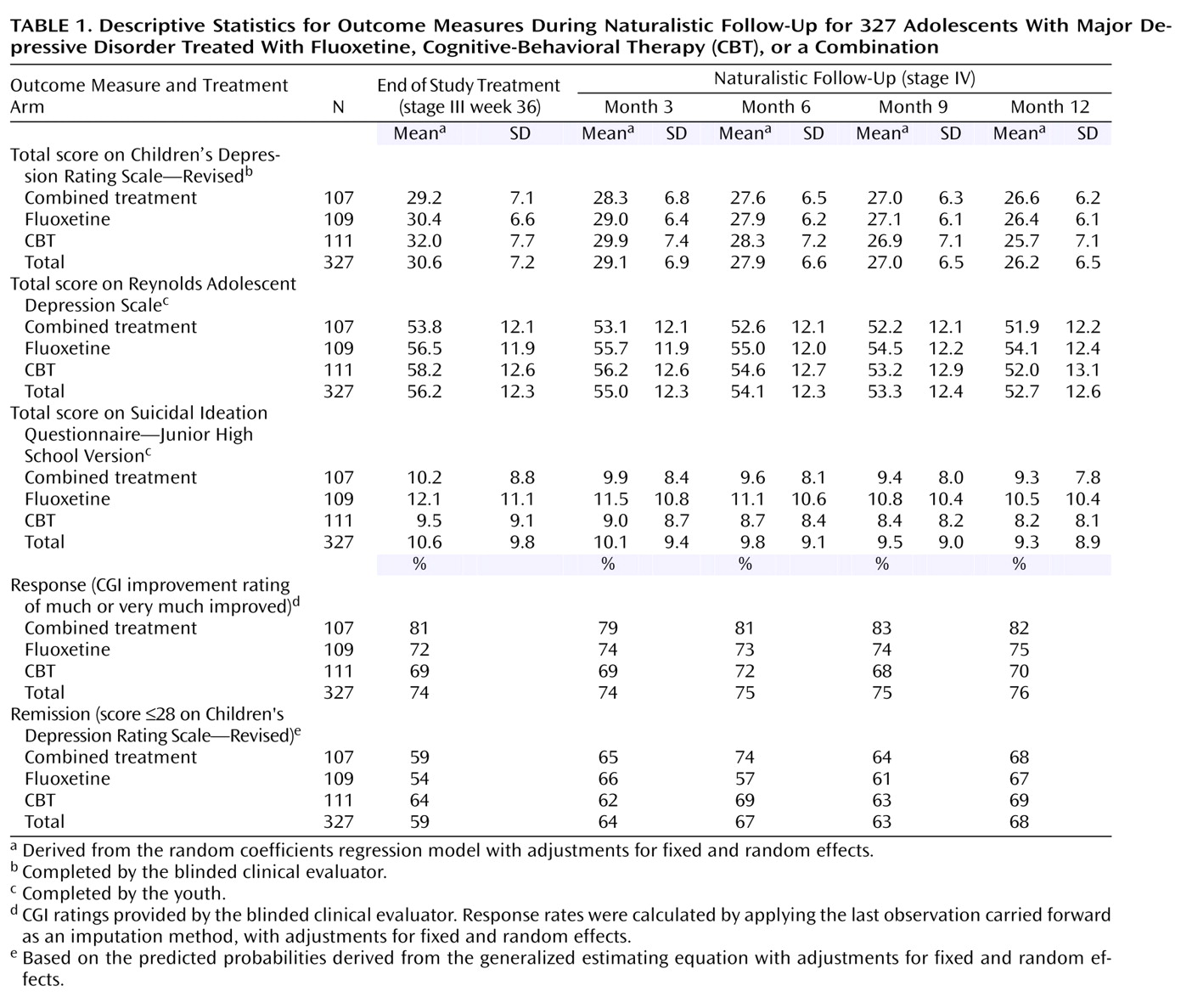
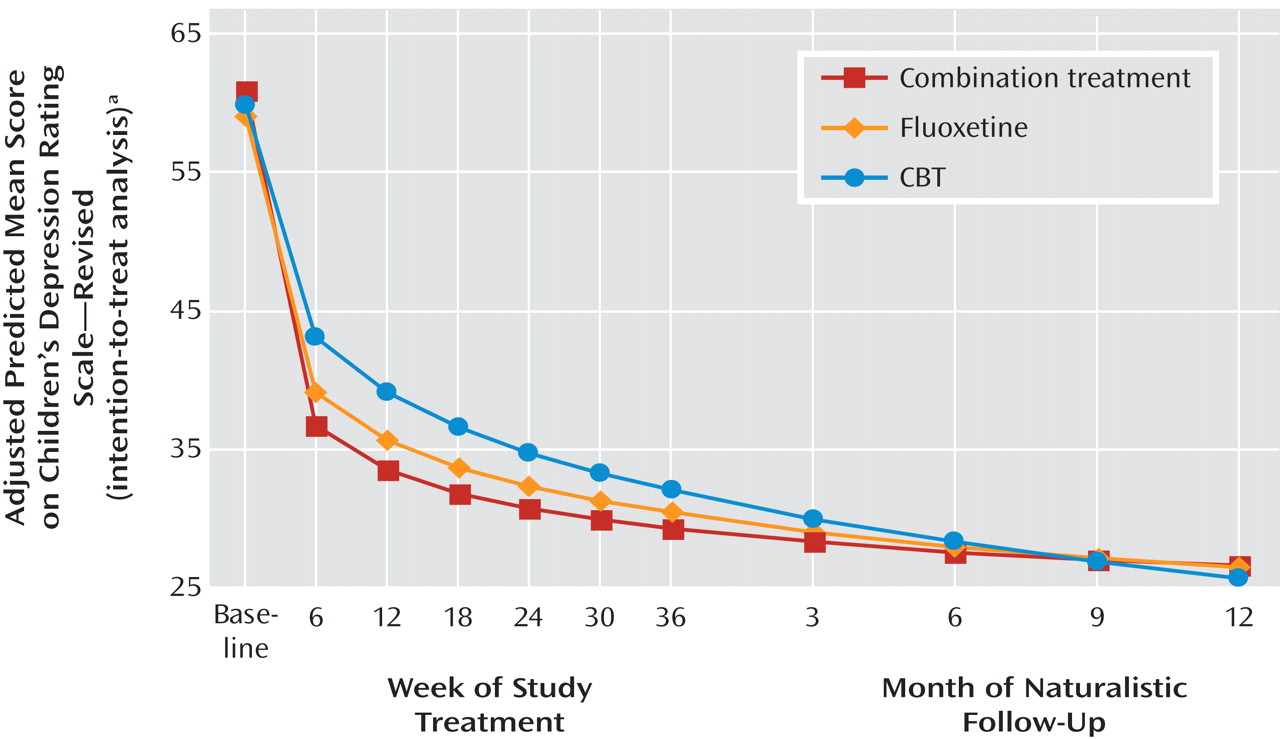
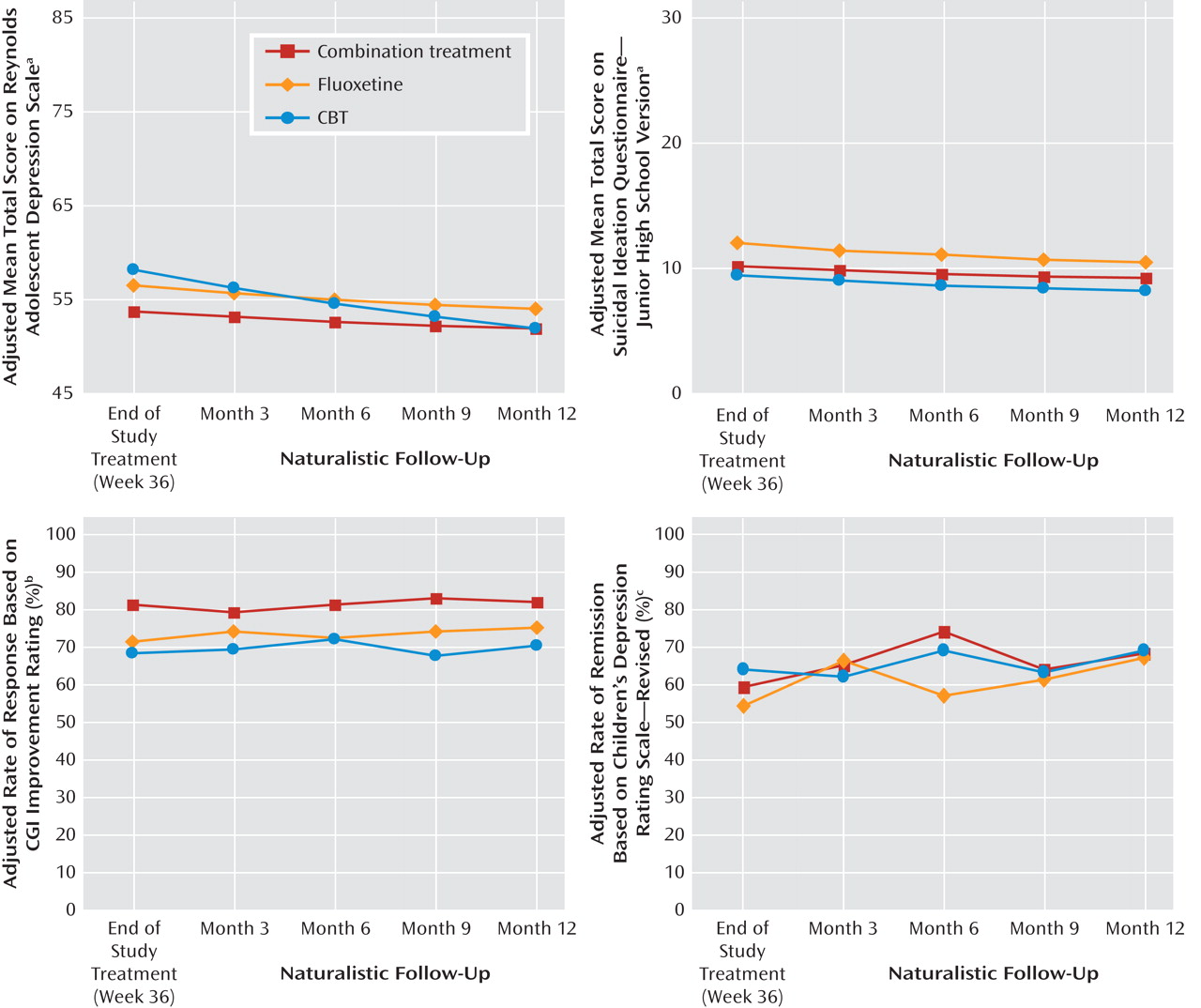
Table 1 presents the adjusted mean scores on the Children’s Depression Rating Scale, Reynolds Adolescent Depression Scale, and Suicidal Ideation Questionnaire, as well as the response and remission rates by the stage IV assessment point for each treatment and the total group.
Figure 1 illustrates the trajectory of scores on the Children’s Depression Rating Scale for the three active treatments across stages I–IV.
Figure 2 illustrates comparable trajectories for the Reynolds Adolescent Depression Scale and Suicidal Ideation Questionnaire, as well as the response and remission measures for the three active treatments across stage IV. When presented in either tabled or graphical format, in all cases the data show that improvement in the group average scores continued on a positive trajectory during stage IV follow-up.
The random regression analyses on the Children’s Depression Rating Scale total scores across the entire study identified a statistically significant linear time effect (F=202.07, df=1, 292, p<0.001), time-by-treatment interaction (F=12.13, df=2, 292, p<0.001), and quadratic time-by-treatment interaction (F=11.72, df=1, 264, p<0.001). The effect of site was significant (F=2.46, df=12, 311, p=0.005). Planned contrasts at week 36 and at stage IV months 3, 6, 9, and 12 identified superiority for combined treatment relative to CBT at week 36 (F=5.08, df=1, 267, p=0.03); all of the stage IV contrasts were nonsignificant.
With a positive response defined as a CGI improvement score of 1 (very much improved) or 2 (much improved), logistic regression using the last observation carried forward indicated that the effect of treatment on the response rate at stage IV month 12 was not statistically significant (χ 2 =4.20, df=2, p=0.13), nor was site (χ 2 =9.70, df=9, p=0.38). The adjusted rates of response at month 12 were 82.2% for combined treatment, 75.2% for fluoxetine, and 70.3% for CBT. Planned contrasts at month 12 identified no significant differences among the treatment groups.
When a cutoff score of 28 or less on the Children’s Depression Rating Scale was used to define remission, generalized estimating equation analyses indicated that the effect of time (χ 2 =123.25, df=8, p<0.001) and the treatment-by-time interaction (χ 2 =30.67, df=16, p=0.02) were statistically significant; the effect of site also was significant (χ 2 =25.09, df=9, p=0.003). All but one of the planned stage IV contrasts were nonsignificant; combined treatment was superior to fluoxetine at month 6 (χ 2 =4.15, df=1, p=0.05).
Random regression analyses of the Reynolds Adolescent Depression Scale total score across time identified a statistically significant linear time effect (F=75.87, df=1, 286, p<0.001), time-by-treatment interaction (F=13.34, df=2, 286, p<0.001), and quadratic time-by-treatment interaction (F=11.60, df=2, 261, p<0.001). The effect of site was significant (F=2.62, df=12, 314, p=0.002). Planned contrasts at weeks 36 and stage IV months 3, 6, 9, and 12 identified superiority for combined treatment relative to CBT at week 36 (F=5.22, df=1, 282, p=0.03); all other stage IV contrasts were nonsignificant.
Random regression analyses of the Suicidal Ideation Questionnaire total score across time identified a statistically significant linear time effect (F=49.53, df=1, 273, p<0.001), quadratic time effect (F=4.53, df=1, 228, p<0.04), and time-by-treatment interaction (F=3.93, df=2, 273, p<0.03). The effect of site was nonsignificant. Planned contrasts at weeks 36 and stage IV months 3, 6, 9, and 12 were nonsignificant.
Loss of Benefits
To evaluate the extent to which subjects deteriorated during stage IV, we examined worsening on the CGI severity scale, Suicidal Ideation Questionnaire, and remission measure. According to the CGI severity rating at week 36, 81.7% of the study group were minimally impaired (CGI score ≤3). During stage IV, 13.1% deteriorated to mildly to extremely impaired (score=4–7): 15.1% in combined treatment, 9.9% in the fluoxetine group, and 14.5% in the CBT group. There were no statistically significant differences across the three treatment groups. According to the suicidality flag, defined as a total score of 31 or greater on the Suicidal Ideation Questionnaire, 91.1% of the sample did not report clinically significant suicidality at week 36. During stage IV, 6.4% developed clinically significant suicidality: 5.9% of those in combined treatment, 7.6% in the fluoxetine group, and 6.4% in the CBT group. There were no statistically significant differences across the three treatment groups. Using a score of 28 or less on the Children’s Depression Rating Scale as the remission threshold, we found that 59.9% of the subjects were in remission at week 36. During stage IV, 32.8% of those in combined treatment, 28.6% of the fluoxetine group, and 30.4% of the CBT group lost remission status at some point. There were no statistically significant differences across the three treatment groups.