Several studies have examined the neural circuitry mediating face-emotion processing in bipolar disorder (
2–
6) and childhood psychopathology (
7–13). For both pediatric and adult bipolar disorder, amygdala dysfunction is perhaps the most commonly reported finding in functional magnetic resonance imaging (fMRI) studies of the illness, with amygdala hyperactivity being reported in a variety of paradigms involving face emotions (
2,
3,
6,
14). Of particular note, Pavuluri et al. (
10,
12) found increased amygdala activation during face-emotion processing in pediatric bipolar disorder. In the present study, we focused on neutral faces because we previously found that 1) children with bipolar disorder rate neutral faces as more hostile and fear producing than comparison subjects, and 2) these differences are associated with amygdala hyperactivity (
11). When viewing neutral faces that transform gradually into an emotional expression, patients with bipolar disorder and severe mood dysregulation require more intense emotional information than comparison subjects in order to identify the expression (
15). Although patients with bipolar disorder or severe mood dysregulation both have impairments in face-emotion labeling, they differ in clinical presentation (
16), outcome (
17–
20), family history (
21), and psychophysiological correlates of frustration (
22). Therefore, one important question is the extent to which neural circuitry mediating face-emotion processing of neutral faces differs between bipolar disorder and severe mood dysregulation.
Given the high rates of ADHD in children with bipolar disorder and severe mood dysregulation (
16), nonirritable children with ADHD are an important comparison group. Emotion regulation and face-emotion labeling deficits emerge in some (
24–
28) but not all (
29) studies of ADHD. Studies in pediatric disruptive behavior disorders have found biased threat appraisal, manifested by subjects rating neutral or ambiguous social situations as affectively negative (
30,
31). Structural and functional imaging studies report amygdala dysfunction in ADHD (
32,
33) (e.g., hyperactivity during reward processing in adults with ADHD [
34]).
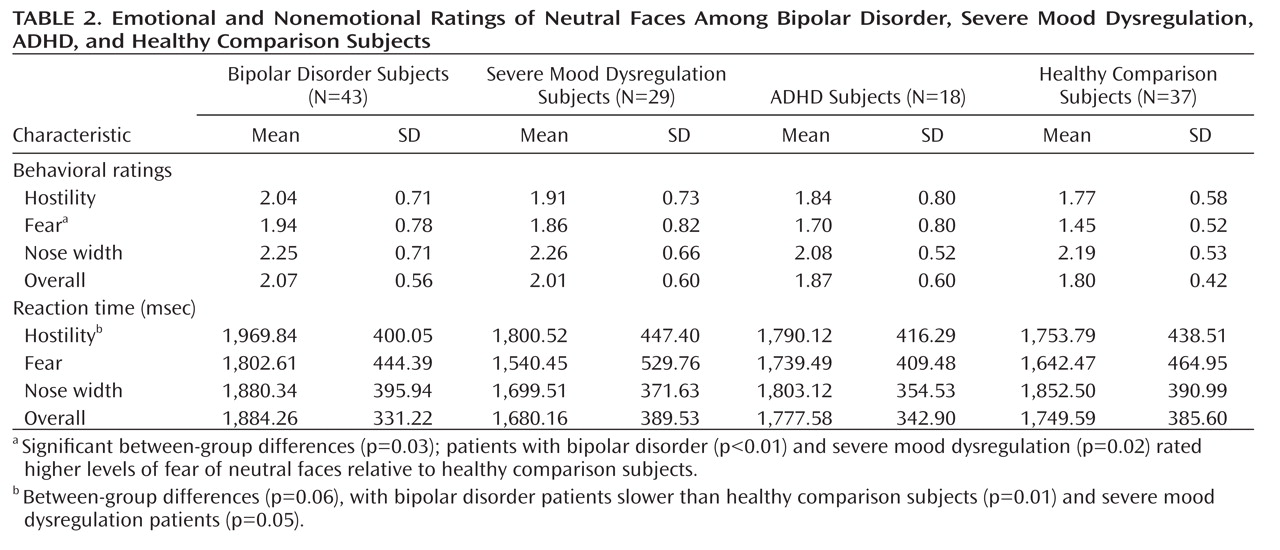
Employing a face-emotion processing task, we compared amygdala perturbations among children with bipolar disorder, ADHD, and severe mood dysregulation as well as healthy comparison subjects. Specifically, subjects made emotional or nonemotional ratings of neutral faces. The contrasts of interest compared neural activity during fear ratings with that of nose-width ratings and neural activity during hostility ratings with that of nose-width ratings (
11). Based on previous work using these contrasts (
11), we hypothesized that relative to healthy comparison subjects, children with bipolar disorder would demonstrate amygdala hyperactivity during emotional (fear or hostility) as opposed to nonemotional (nose-width) ratings of neutral faces. Children with severe mood dysregulation also have deficits in face-emotion labeling (
29), including on a behavioral paradigm that involves neutral faces (
15). Data suggest that this form of mood dysregulation is a risk factor for depressive disorders (
17–
20), and two studies suggest that youths with major depressive disorder exhibit amygdala hypoactivation when viewing faces in some contexts (
9,
13). Accordingly, we hypothesized that children with severe mood dysregulation would show decreased amygdala activation while rating emotional or nonemotional aspects of neutral faces. No prior studies, to our knowledge, have examined amygdala response to emotional and nonemotional ratings of faces in ADHD patients, and behavioral results in face-emotion labeling studies are inconsistent (
24–
29). Therefore, while we expected functional amygdala perturbation in patients with ADHD, data to generate specific hypotheses are insufficient.
Discussion
It is important to examine phenotypic entities with overlapping clinical features to determine similarities and differences in neural perturbations. Face-emotion processing is a salient feature of social cognition and is deficient in several childhood pathologies, including bipolar disorder, severe mood dysregulation, and, possibly, ADHD (
25–
29). We compared amygdala activation during a face-emotion processing task in youths with ADHD, bipolar disorder, or severe mood dysregulation as well as healthy comparison subjects (i.e., subjects with no axis I diagnosis). This is the first study, to our knowledge, to compare amygdala activation in these clinically overlapping groups using a face-emotion processing paradigm and the first fMRI study to examine face processing in severe mood dysregulation.
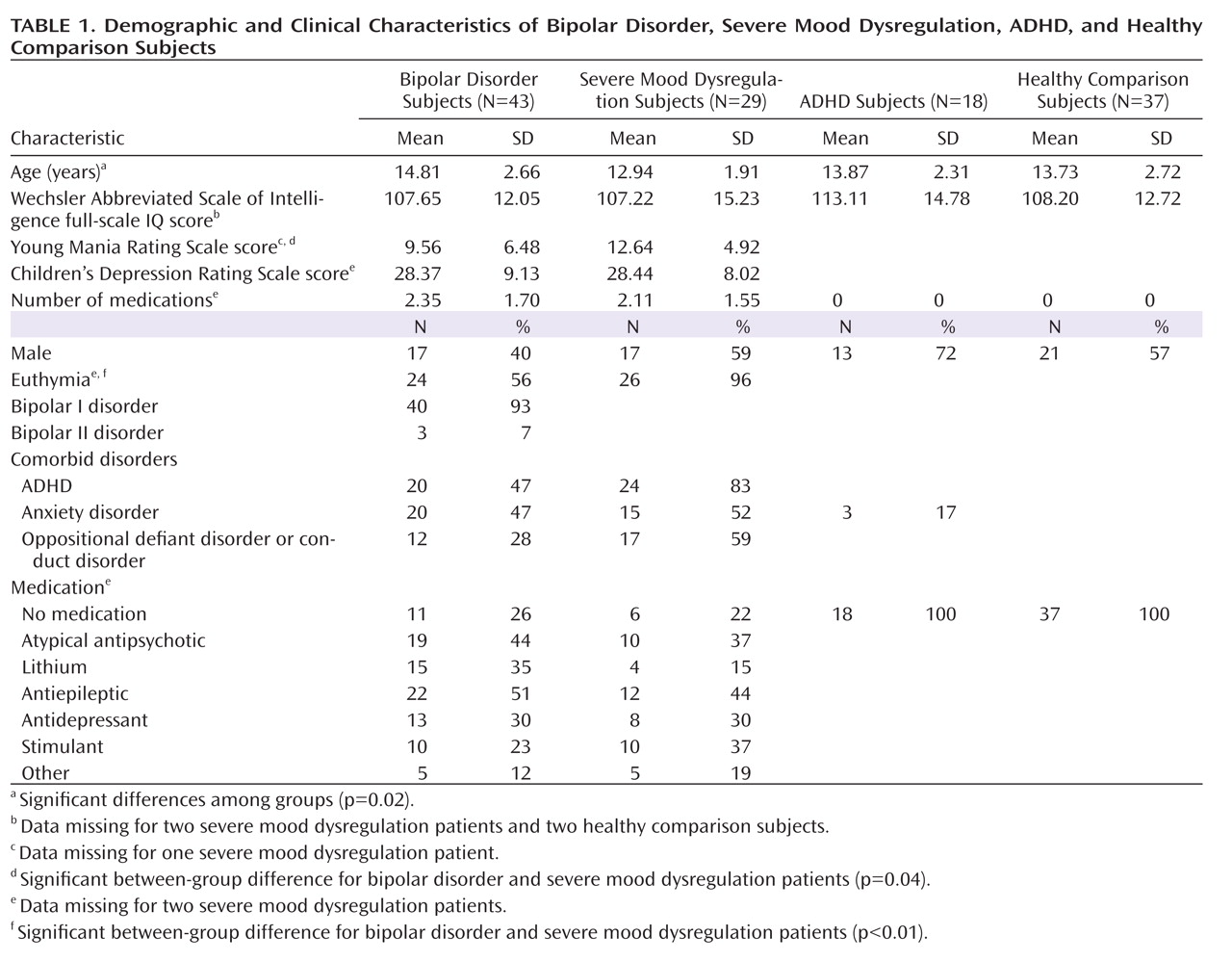
We found an imaging-based double dissociation in amygdala activation in patients with ADHD and severe mood dysregulation. Nonirritable ADHD youths showed amygdala hyperactivity when completing subjective fear ratings of neutral faces relative to healthy youths and those with bipolar disorder or severe mood dysregulation. In contrast, children with chronic irritability (operationalized using Leibenluft et al.'s criteria for severe mood dysregulation [
1]) showed amygdala hypoactivity relative to healthy youths and those with bipolar disorder or ADHD. Contrary to our hypothesis, patients with bipolar disorder did not differ significantly from healthy comparison subjects. These findings suggest that there may be functional differences among ADHD, bipolar disorder, and severe mood dysregulation patients, despite the presence of overlapping behavioral deficits and clinical symptoms.
Severe mood dysregulation is characterized by severe, nonepisodic irritability and hyperarousal. Although youths with severe mood dysregulation typically meet criteria for ADHD (
16) and are often assigned the diagnosis of bipolar disorder, they may be seen as clinically "in between" these two groups. Unlike ADHD, severe mood dysregulation is characterized by a distinct and highly impairing mood component. However, in contrast to bipolar disorder, it does not involve discrete hypomanic or manic episodes. Several studies indicate that similar to youths with bipolar disorder, youths with severe mood dysregulation are deficient in the ability to identify and label facial emotions (
15,
29), which is consistent with the behavioral and neural findings in the present study. When performing emotional ratings (i.e., subjective fear) of neutral faces, severe mood dysregulation patients exhibited reduced activity in the amygdala relative to healthy comparison subjects and patients with bipolar disorder or ADHD. The amygdala mediates emotional processing and valence (
43) and is involved in the processing of facial affect (
44). In severe mood dysregulation, the deficit in amygdala engagement while processing emotional aspects of facial expressions could contribute to interpersonal difficulties and mood problems.
Our finding in severe mood dysregulation resembles data reported for youths with major depressive disorder. Using this same task, viewing both fearful and neutral faces, Beesdo et al. (
9) found amygdala hypoactivation in children with major depressive disorder. Similarities between fMRI findings in severe mood dysregulation and major depressive disorder are interesting because longitudinal epidemiological research suggests that severe mood dysregulation, and chronic irritability in general, are associated with subsequent depressive disorders (
17–
20,
45). Thus, both longitudinal and neuroimaging data suggest associations between severe mood dysregulation and major depressive disorder. Pathophysiological similarities between these two disorders merit further investigation, with a particular emphasis on whether amygdala dysfunction in severe mood dysregulation predicts later major depressive disorder. In addition, the psychological and physiological underpinnings of amygdala hypoactivation in severe mood dysregulation during fear versus nose-width ratings of neutral faces warrant further exploration. For example, our data suggest that this finding could, in part, reflect relative hyperactivation in youths with severe mood dysregulation, compared with other youths, while rating nose width, perhaps because youths with this disorder have difficulty attending away from face emotions. Alternatively, it could reflect perturbations in other baseline conditions, given questions regarding the appropriate baseline in fMRI (
46).
Given the high rate of ADHD in youths with severe mood dysregulation (
16), it is interesting that the neural correlates of face-emotion processing differ markedly between these two patient groups. Although both severe mood dysregulation and ADHD patients have symptoms of distractibility and increased motor activity, they differ in that patients with severe mood dysregulation, but not ADHD, have significant irritability. Using the K-SADS-PL, with an additional severe mood dysregulation module, the ADHD subjects in the present sample were evaluated carefully to ensure that they did not have significant irritability or other mood symptoms. Thus, it is somewhat surprising that the ADHD children demonstrated amygdala hyperactivity during a face processing task. However, studies demonstrate structural (
32) and dopaminergic (
33) abnormalities in the amygdala in ADHD patients, suggesting that amygdala abnormalities in ADHD are not without precedent. In the only previous fMRI study of face processing in ADHD, Marsh et al. (
47), employing a partially overlapping ADHD group, found that amygdala activity did not differ between ADHD patients and comparison subjects. The task used by Marsh et al. and the one used in the present study differ significantly in attentional demands (i.e., implicit versus explicit processing), possibly accounting for the disparate results.
Using a subset of the present sample, we previously found increased activation in the left amygdala in bipolar disorder patients relative to healthy comparison subjects in the hostility and subjective fear conditions (
11). In both our previous study (
11) and the present study, bipolar disorder patients, relative to healthy comparison subjects, rated neutral faces as more fear producing and exhibited slower reaction times when rating face hostility. Our failure to extend this previous neuroimaging finding might be the result of a type II error, particularly since the current analysis includes four groups rather than two. Indeed, this face-viewing paradigm is particularly prone to type II error because it includes multiple face emotions and attention states, each sampled relatively sparsely (eight replicates per attentional condition). Such sparse sampling was necessary to maintain short task duration and increase tolerability for youths with severe psychopathology.
In addition, we were interested in a task that included neutral faces, given our prior behavioral and imaging results (
11, 15). However, in examining responses to neutral faces, it is important to embed them in other face emotions, since other emotions can affect the interpretation of neutral faces (
48). Thus, our task represents a compromise. We sampled activation when neutral faces were viewed in multiple attention states against a background of multiple emotions, but the number of trials for neutral faces in each attention state was relatively low.
Indeed, it is possible that the presence of other emotions may have influenced our findings of amygdala activity in response to neutral faces. Although all subjects were exposed to the same number of emotional facial expressions, one group may have been particularly affected by exposure to angry and/or fearful faces, and this, in turn, may have influenced the group's processing of neutral faces. Research in amygdala dysfunction in pediatric bipolar disorder could use more powerful paradigms, perhaps focused specifically on emotional ratings of neutral faces or on other face emotions, such as fear or anger (
49).
Additional limitations complicate interpretations. First, most bipolar disorder and severe mood dysregulation youths were medicated. However, prior work suggests that medications do not typically cause type I errors and may even diminish between-group differences (
50). Youths with ADHD but not bipolar disorder or severe mood dysregulation were withdrawn from stimulants before scanning. The influence of recent medication withdrawal on activation remains unknown. Second, some bipolar disorder and severe mood dysregulation patients were not experiencing a period of euthymia at the time of testing, and negative affect on the day of the scan was not assessed in the ADHD group. Third, age differed among the groups, although analyses controlled for this difference. Fourth, we used standard anatomical criteria to locate the amygdala boundaries. Since studies report abnormalities in amygdala structure in patients with bipolar disorder and ADHD (
32,
51), standard anatomical criteria may not be the most accurate method of identifying this region of interest. Future research might trace the individual amygdala for each subject, although this may become infeasible as studies continue to increase in size.
The present study suggests unique neural mechanisms mediating face-emotion processing deficits in clinically overlapping groups. There was a double dissociation in amygdala activity in youths with ADHD and severe mood dysregulation when completing fear (emotional) versus nonemotional ratings of neutral faces. ADHD patients demonstrated hyperactivation, while patients with severe mood dysregulation demonstrated hypoactivation, relative to the other groups. Additional neuroimaging studies are needed to examine amygdala activity in response to other emotional facial expressions in these youths and further specify the neural perturbations associated with nosologically and phenotypically similar and distinct clinical entities.