To the Editor: We have been conducting a 5-year study, funded by the National Institute of Mental Health, to compare four commonly used atypical antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone) in middle-aged and older patients with psychotic symptoms for whom a treating clinician has recommended an atypical antipsychotic. In this Institutional Review Board-approved protocol, we use equipoise-stratified randomization
(1), which allows the patients and their physicians to exclude up to two of these antipsychotic agents that are not acceptable to them. The treating physician chooses the dosage and duration of treatment. The detailed study design is described elsewhere
(2) . We report the results of an unplanned interim analysis recommended by our Data and Safety Monitoring Board, which led to the discontinuation of quetiapine from the study. It includes the first 294 of the proposed 450 subjects to be enrolled. Sixty-one subjects dropped out prior to data collection and were excluded from analysis. Diagnoses for the remaining 233 patients were as follows: schizophrenia (32%); bipolar disorder (11%); and psychosis associated with dementia (24%), with depression (11%), with posttraumatic stress disorder (16%), or not otherwise specified (6%).
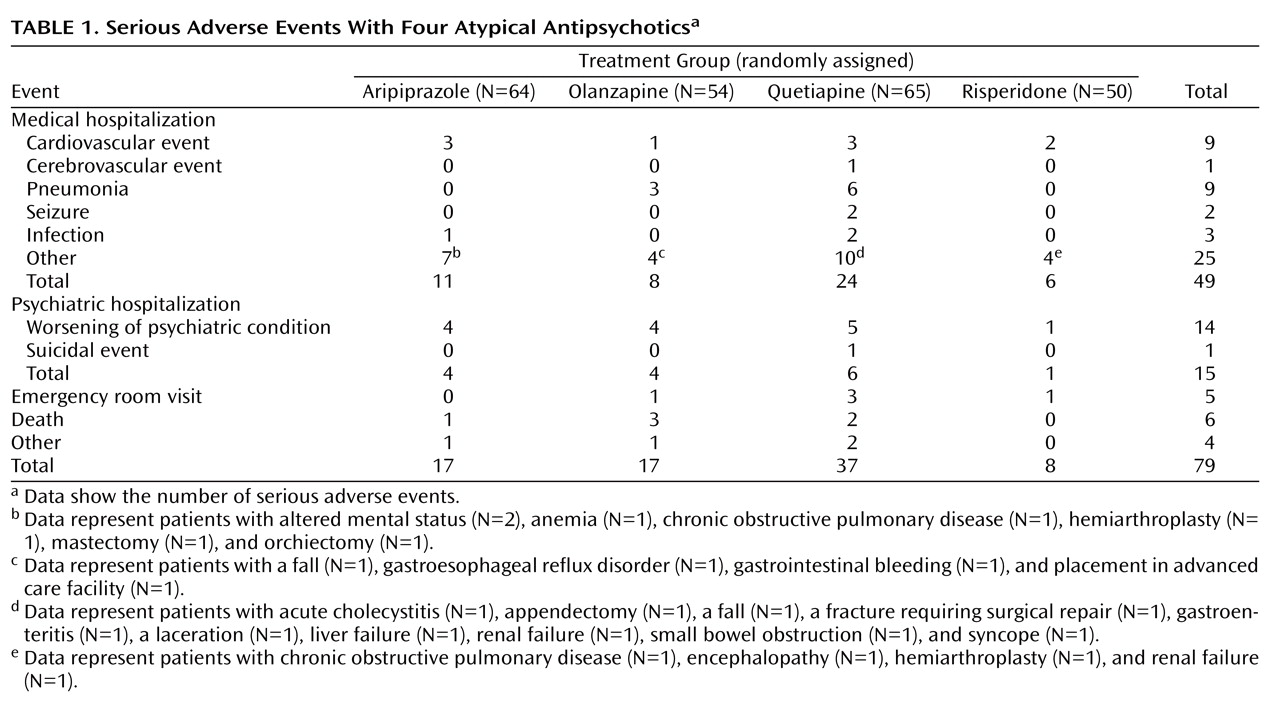
There were 79 Food and Drug Administration-defined serious adverse events
(3) that occurred in 57 of the 233 study subjects while they were taking their randomly assigned drugs (
Table 1 ). Among the patients taking quetiapine, 38.5% had serious adverse events relative to 19.0% in other groups (χ
2 =9.56, df=1, p=0.002), providing a relative risk of 2.0 (confidence interval=1.3–3.1). All pairwise differences involving quetiapine were significant. Using Mantel-Haenszel tests to account for the equipoise-stratified randomization and pooling the other drug groups, the quetiapine group exhibited higher rates of serious adverse events (χ
2 =5.63, df=1, p=0.022). (Pairwise differences using Mantel-Haenszel analysis did not reach significance.) Of the serious adverse events with quetiapine, 29.7% (11/37) were rated as “probably” or “possibly” related to the medication. For other drugs, 21.1% (9/42) of the serious adverse events were rated as “probably” or “possibly” related to the medication. Of the individual types of medical serious adverse events, rates of pneumonia were higher with quetiapine than with other drugs combined (p=0.011, Fisher’s exact).
A multivariate logistic regression analysis for all serious adverse events was performed, covarying for age, prior antipsychotic treatment, and medical burden. The overall difference in the proportion of serious adverse events among the four drug groups remained similar in all analyses. Likewise, differences in length of treatment did not seem to be responsible for differential rates of serious adverse events, since there was no significant difference between quetiapine and other drugs regarding the duration the subjects continued to take their randomly assigned medication.